BAFF knockout improves systemic inflammation via regulating adipose tissue distribution in high-fat diet-induced obesity
- PMID: 25591987
- PMCID: PMC4314587
- DOI: 10.1038/emm.2014.98
BAFF knockout improves systemic inflammation via regulating adipose tissue distribution in high-fat diet-induced obesity
Abstract
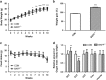
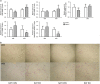
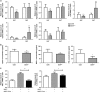
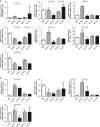
Obesity is recognized as a chronic low-grade inflammatory state due to adipose tissue expansion being accompanied by an increase in the production of proinflammatory adipokines. Our group is the first to report that B-cell-activating factor (BAFF) is produced from adipocytes and functions as a proinflammatory adipokine. Here, we investigated how loss of BAFF influenced diet-induced obesity in mice by challenging BAFF(-/-) mice with a high-fat diet for 10 weeks. The results demonstrated that weight gain in BAFF(-/-) mice was >30% than in control mice, with a specific increase in the fat mass of the subcutaneous region rather than the abdominal region. Expression of lipogenic genes was examined by quantitative real-time PCR, and increased lipogenesis was observed in the subcutaneous adipose tissue (SAT), whereas lipogenesis in the epididymal adipose tissue (EAT) was reduced. A significant decrease in EAT mass resulted in the downregulation of inflammatory gene expression in EAT, and more importantly, overall levels of inflammatory cytokines in the circulation were reduced in obese BAFF(-/-) mice. We also observed that the macrophages recruited in the enlarged SAT were predominantly M2 macrophages. 3T3-L1 adipocytes were cultured with adipose tissue conditioned media (ATCM), demonstrating that EAT ATCM from BAFF(-/-) mice contains antilipogenic and anti-inflammatory properties. Taken together, BAFF(-/-) improved systemic inflammation by redistributing adipose tissue into subcutaneous regions. Understanding the mechanisms by which BAFF regulates obesity in a tissue-specific manner would provide therapeutic opportunities to target obesity-related chronic diseases.
Figures

References
-
- Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004;4:579–591. - PubMed
-
- Dandona P, Aljada A, Chaudhuri A, Mohanty P, Garg R. Metabolic syndrome: a comprehensive perspective based on interactions between obesity, diabetes, and inflammation. Circulation. 2005;111:1448–1454. - PubMed
-
- Grundy SM. Obesity, metabolic syndrome, and cardiovascular disease. J Clin Endocrinol Metab. 2004;89:2595–2600. - PubMed
-
- Fantuzzi G. Adipose tissue, adipokines, and inflammation. J Allergy Clin Immunol. 2005;115:911–919. - PubMed
-
- Wisse BE. The inflammatory syndrome: the role of adipose tissue cytokines in metabolic disorders linked to obesity. J Am Soc Nephrol. 2004;15:2792–2800. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

