Intraocular pharmacokinetics of intravitreal vascular endothelial growth factor-Trap in a rabbit model
- PMID: 25592118
- PMCID: PMC4816356
- DOI: 10.1038/eye.2014.329
Intraocular pharmacokinetics of intravitreal vascular endothelial growth factor-Trap in a rabbit model
Abstract
Purpose: To determine intraocular pharmacokinetic properties of intravitreally injected vascular endothelial growth factor (VEGF)-Trap in a rabbit model.
Methods: VEGF-Trap was intravitreally injected in 18 rabbit eyes. Eyes were enucleated 1 h and 1, 2, 5, 14, and 30 days after injections and immediately frozen at -80 °C. Concentration of VEGF-Trap in vitreous, aqueous humor, and retina/choroid was determined using an indirect enzyme-linked immunosorbent assay and analyzed to obtain pharmacokinetic properties.
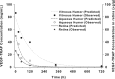
Results: Maximum concentration of VEGF-Trap was achieved at 1 h in all three tissues. A one-compartment model of distribution was selected as the final model for all tissues studied. Estimated half-life of VEGF-Trap in vitreous, aqueous humor, and retinal/choroid was 87.1, 36.8, and 35.0 h, respectively, and estimated mean residence time was 125.7, 53.1, and 50.5 h, respectively. Area under the curve from time 0 to the end point was 10009.8, 3945.1, and 1189.3, respectively. Total exposure of the aqueous humor and retina/choroid to VEGF-Trap was 39.4% and 11.9% of vitreous exposure, respectively.
Conclusion: The vitreous half-life of VEGF-Trap is 3.63 days. This is shorter than that of bevacizumab (6.99 days) and longer than that of ranibizumab (2.51 days), as shown in studies using the same experimental settings. The concentration of VEGF-Trap peaked at 1 h after injections in all eye tissues studied.
Figures


References
-
- Brown DM, Kaiser PK, Michels M, Soubrane G, Heier JS, Kim RY et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med 2006; 355: 1432–1444. - PubMed
-
- Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med 2006; 355: 1419–1431. - PubMed
-
- Campochiaro PA, Heier JS, Feiner L, Gray S, Saroj N, Rundle AC et al. Ranibizumab for macular edema following branch retinal vein occlusion: six-month primary end point results of a phase III study. Ophthalmology 2010; 117: 1102–1112 e1101. - PubMed
-
- Nguyen QD, Brown DM, Marcus DM, Boyer DS, Patel S, Feiner L et al. Ranibizumab for diabetic macular edema: results from 2 phase iii randomized trials: RISE and RIDE. Ophthalmology 2012; 119(4): 789–801. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

