Rituximab in Children with Steroid-Dependent Nephrotic Syndrome: A Multicenter, Open-Label, Noninferiority, Randomized Controlled Trial
- PMID: 25592855
- PMCID: PMC4552120
- DOI: 10.1681/ASN.2014080799
Rituximab in Children with Steroid-Dependent Nephrotic Syndrome: A Multicenter, Open-Label, Noninferiority, Randomized Controlled Trial
Abstract
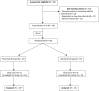
Steroid-dependent nephrotic syndrome (SDNS) carries a high risk of toxicity from steroids or steroid-sparing agents. This open-label, noninferiority, randomized controlled trial at four sites in Italy tested whether rituximab is noninferior to steroids in maintaining remission in juvenile SDNS. We enrolled children age 1-16 years who had developed SDNS in the previous 6-12 months and were maintained in remission with high prednisone doses (≥0.7 mg/kg per day). We randomly assigned participants to continue prednisone alone for 1 month (control) or to add a single intravenous infusion of rituximab (375 mg/m(2); intervention). Prednisone was tapered in both groups after 1 month. For noninferiority, rituximab had to permit steroid withdrawal and maintain 3-month proteinuria (mg/m(2) per day) within a prespecified noninferiority margin of three times the levels among controls (primary outcome). We followed participants for ≥1 year to compare risk of relapse (secondary outcome). Fifteen children per group (21 boys; mean age, 7 years [range, 2.6-13.5 years]) were enrolled and followed for ≤60 months (median, 22 months). Three-month proteinuria was 42% lower in the rituximab group (geometric mean ratio, 0.58; 95% confidence interval, 0.18 to 1.95 [i.e., within the noninferiority margin of three times the levels in controls]). All but one child in the control group relapsed within 6 months; median time to relapse in the rituximab group was 18 months (95% confidence interval, 9 to 32 months). In the rituximab group, nausea and skin rash during infusion were common; transient acute arthritis occurred in one child. In conclusion, rituximab was noninferior to steroids for the treatment of juvenile SDNS.
Keywords: nephrotic syndrome; primary glomerulonephritis; randomized controlled trials.
Copyright © 2015 by the American Society of Nephrology.
Figures




Comment in
-
Nephrotic syndrome: Efficacy of rituximab in challenging nephrotic syndrome.Nat Rev Nephrol. 2015 May;11(5):257-8. doi: 10.1038/nrneph.2015.26. Epub 2015 Mar 10. Nat Rev Nephrol. 2015. PMID: 25752834
References
-
- Radhakrishnan J, Cattran DC: The KDIGO practice guideline on glomerulonephritis: reading between the (guide)lines—application to the individual patient. Kidney Int 82: 840–856, 2012 - PubMed
-
- Korbet SM, Schwartz MM, Lewis EJ: Primary focal segmental glomerulosclerosis: Clinical course and response to therapy. Am J Kidney Dis 23: 773–783, 1994 - PubMed
-
- McEnery PT, Strife CF: Nephrotic syndrome in childhood. Management and treatment in patients with minimal change disease, mesangial proliferation, or focal glomerulosclerosis. Pediatr Clin North Am 29: 875–894, 1982 - PubMed
-
- Ghiggeri GM, Carraro M, Vincenti F: Recurrent focal glomerulosclerosis in the era of genetics of podocyte proteins: Theory and therapy. Nephrol Dial Transplant 19: 1036–1040, 2004 - PubMed
-
- ISKDC : Primary nephrotic syndrome in children: Clinical significance of histopathologic variants of minimal change and of diffuse mesangial hypercellularity. A report of the International Study of Kidney Disease in Children. Kidney Int 20: 765–771, 1981 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

