From equator to pole: splitting chromosomes in mitosis and meiosis
- PMID: 25593304
- PMCID: PMC4298131
- DOI: 10.1101/gad.255554.114
From equator to pole: splitting chromosomes in mitosis and meiosis
Abstract
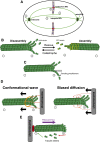
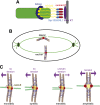
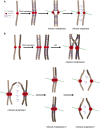
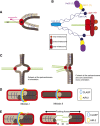
During eukaryotic cell division, chromosomes must be precisely partitioned to daughter cells. This relies on a mechanism to move chromosomes in defined directions within the parental cell. While sister chromatids are segregated from one another in mitosis and meiosis II, specific adaptations enable the segregation of homologous chromosomes during meiosis I to reduce ploidy for gamete production. Many of the factors that drive these directed chromosome movements are known, and their molecular mechanism has started to be uncovered. Here we review the mechanisms of eukaryotic chromosome segregation, with a particular emphasis on the modifications that ensure the segregation of homologous chromosomes during meiosis I.
Keywords: kinetochore; meiosis; microtubules; mitosis.
© 2015 Duro and Marston; Published by Cold Spring Harbor Laboratory Press.
Figures
References
-
- Albertson D, Thomson JN. 1993. Segregation of holocentric chromosomes at meiosis in the nematode, Caenorhabditis elegans. Chromosome Res 1: 15–26. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
