Genome-wide chromatin footprinting reveals changes in replication origin architecture induced by pre-RC assembly
- PMID: 25593310
- PMCID: PMC4298139
- DOI: 10.1101/gad.247924.114
Genome-wide chromatin footprinting reveals changes in replication origin architecture induced by pre-RC assembly
Abstract
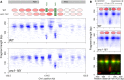
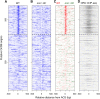
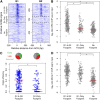
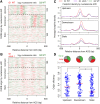
Start sites of DNA replication are marked by the origin recognition complex (ORC), which coordinates Mcm2-7 helicase loading to form the prereplicative complex (pre-RC). Although pre-RC assembly is well characterized in vitro, the process is poorly understood within the local chromatin environment surrounding replication origins. To reveal how the chromatin architecture modulates origin selection and activation, we "footprinted" nucleosomes, transcription factors, and replication proteins at multiple points during the Saccharomyces cerevisiae cell cycle. Our nucleotide-resolution protein occupancy profiles resolved a precise ORC-dependent footprint at 269 origins in G2. A separate class of inefficient origins exhibited protein occupancy only in G1, suggesting that stable ORC chromatin association in G2 is a determinant of origin efficiency. G1 nucleosome remodeling concomitant with pre-RC assembly expanded the origin nucleosome-free region and enhanced activation efficiency. Finally, the local chromatin environment restricts the loading of the Mcm2-7 double hexamer either upstream of or downstream from the ARS consensus sequence (ACS).
Keywords: DNA replication; chromatin; nucleosome; origin recognition complex (ORC).
© 2015 Belsky et al.; Published by Cold Spring Harbor Laboratory Press.
Figures
References
-
- Aparicio OM, Weinstein DM, Bell SP. 1997. Components and dynamics of DNA replication complexes in S. cerevisiae: redistribution of MCM proteins and Cdc45p during S phase. Cell 91: 59–69. - PubMed
-
- Bai L, Morozov AV. 2010. Gene regulation by nucleosome positioning. Trends Genet 26: 476–483. - PubMed
-
- Bell SP, Stillman B. 1992. ATP-dependent recognition of eukaryotic origins of DNA replication by a multiprotein complex. Nature 357: 128–134. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
