A systematic survey of the Cys2His2 zinc finger DNA-binding landscape
- PMID: 25593323
- PMCID: PMC4330361
- DOI: 10.1093/nar/gku1395
A systematic survey of the Cys2His2 zinc finger DNA-binding landscape
Abstract
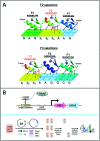
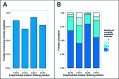
Cys2His2 zinc fingers (C2H2-ZFs) comprise the largest class of metazoan DNA-binding domains. Despite this domain's well-defined DNA-recognition interface, and its successful use in the design of chimeric proteins capable of targeting genomic regions of interest, much remains unknown about its DNA-binding landscape. To help bridge this gap in fundamental knowledge and to provide a resource for design-oriented applications, we screened large synthetic protein libraries to select binding C2H2-ZF domains for each possible three base pair target. The resulting data consist of >160 000 unique domain-DNA interactions and comprise the most comprehensive investigation of C2H2-ZF DNA-binding interactions to date. An integrated analysis of these independent screens yielded DNA-binding profiles for tens of thousands of domains and led to the successful design and prediction of C2H2-ZF DNA-binding specificities. Computational analyses uncovered important aspects of C2H2-ZF domain-DNA interactions, including the roles of within-finger context and domain position on base recognition. We observed the existence of numerous distinct binding strategies for each possible three base pair target and an apparent balance between affinity and specificity of binding. In sum, our comprehensive data help elucidate the complex binding landscape of C2H2-ZF domains and provide a foundation for efforts to determine, predict and engineer their DNA-binding specificities.
© The Author(s) 2015. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

