Design and implementation of the START (STem cells for ARDS Treatment) trial, a phase 1/2 trial of human mesenchymal stem/stromal cells for the treatment of moderate-severe acute respiratory distress syndrome
- PMID: 25593740
- PMCID: PMC4273700
- DOI: 10.1186/s13613-014-0022-z
Design and implementation of the START (STem cells for ARDS Treatment) trial, a phase 1/2 trial of human mesenchymal stem/stromal cells for the treatment of moderate-severe acute respiratory distress syndrome
Abstract
Background: Despite advances in supportive care, moderate-severe acute respiratory distress syndrome (ARDS) is associated with high mortality rates, and novel therapies to treat this condition are needed. Compelling pre-clinical data from mouse, rat, sheep and ex vivo perfused human lung models support the use of human mesenchymal stem (stromal) cells (MSCs) as a novel intravenous therapy for the early treatment of ARDS.
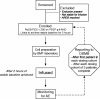
Methods: This article describes the study design and challenges encountered during the implementation and phase 1 component of the START (STem cells for ARDS Treatment) trial, a phase 1/2 trial of bone marrow-derived human MSCs for moderate-severe ARDS. A trial enrolling 69 subjects is planned (9 subjects in phase 1, 60 subjects in phase 2 treated with MSCs or placebo in a 2:1 ratio).
Results: This report describes study design features that are unique to a phase 1 trial in critically ill subjects and the specific challenges of implementation of a cell-based therapy trial in the ICU.
Conclusions: Experience gained during the design and implementation of the START study will be useful to investigators planning future phase 1 clinical trials based in the ICU, as well as trials of cell-based therapy for other acute illnesses.
Clinical trials registration: NCT01775774 and NCT02097641.
Keywords: Acute lung injury; Clinical trial; Mesenchymal stem/stromal cell; Pulmonary edema.
Figures
References
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical