Endometriosis: a new cellular and molecular genetic approach for understanding the pathogenesis and evolutivity
- PMID: 25593940
- PMCID: PMC4286973
- DOI: 10.3389/fsurg.2014.00016
Endometriosis: a new cellular and molecular genetic approach for understanding the pathogenesis and evolutivity
Abstract
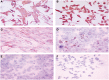
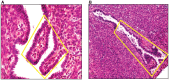
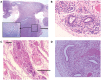
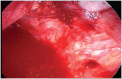
Endometriosis is a benign disease with high prevalence in women of reproductive age estimated between 10 and 15% and is associated with considerable morbidity. Its etiology and pathogenesis are controversial but it is believed to involve multiple genetic, environmental, immunological, angiogenic, and endocrine processes. Altered expressions of growth factors, cytokines, adhesion molecules, matrix metalloproteinases, and enzymes for estrogen synthesis and metabolism have been frequently observed in this condition. The possibility of genetic basis of endometriosis is demonstrated in studies of familial disease, in which the incidence of endometriosis is higher for first-degree relatives of probands as compared to controls. This review describes mainly the cellular, cytochemical, cytogenetic, and molecular genetic features of endometriotic lesions and cultured endometriotic cells. In attempts to identify candidate gene (s) involved in the pathogenesis of endometriosis, a tissue-based approaches including conventional cytogenetics (RHG-banding), loss of heterozygosity (LOH), and comparative genomic hybridization (CGH) were employed. In addition to the karyotypic anomalies, consistent chromosome instability was confirmed by CGH and fluorescence in situ hybridization (FISH). The nature and significance of the molecular genetic aberrations in relation to the locations and function of oncogenes and tumor suppressor genes will be discussed. At last, a possible pathogenic role of embryonic duct remnants was observed in seven female fetal reproductive tract in endometriosis and may induce a discussion about the beginning of ovarian tumors and malignant proliferations.
Keywords: chromosome; comparative genomic hybridization; embryonic duct remnants; endometriosis; fluorescence in situ hybridization; permanent cell line.
Figures





References
-
- Ballouk F, Ross JS, Wolf BC. Ovarian endometriotic cysts: an analysis of cytologic atypia and DNA ploidy. Am J Clin Pathol (1994) 102:415–9. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

