Biology of infantile hemangioma
- PMID: 25593962
- PMCID: PMC4286974
- DOI: 10.3389/fsurg.2014.00038
Biology of infantile hemangioma
Abstract
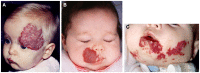
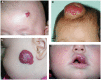
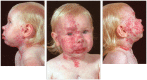
Infantile hemangioma (IH), the most common tumor of infancy, is characterized by an initial proliferation during infancy followed by spontaneous involution over the next 5-10 years, often leaving a fibro-fatty residuum. IH is traditionally considered a tumor of the microvasculature. However, recent data show the critical role of stem cells in the biology of IH with emerging evidence suggesting an embryonic developmental anomaly due to aberrant proliferation and differentiation of a hemogenic endothelium with a neural crest phenotype that possesses the capacity for endothelial, hematopoietic, mesenchymal, and neuronal differentiation. Current evidence suggests a putative placental chorionic mesenchymal core cell embolic origin of IH during the first trimester. This review outlines the emerging role of stem cells and their interplay with the cytokine niche that promotes a post-natal environment conducive for vasculogenesis involving VEGFR-2 and its ligand VEGF-A and the IGF-2 ligand in promoting cellular proliferation, and the TRAIL-OPG anti-apoptotic pathway in preventing cellular apoptosis in IH. The discovery of the role of the renin-angiotensin system in the biology of IH provides a plausible explanation for the programed biologic behavior and the β-blocker-induced accelerated involution of this enigmatic condition. This crucially involves the vasoactive peptide, angiotensin II, that promotes cellular proliferation in IH predominantly via its action on the ATIIR2 isoform. The role of the RAS in the biology of IH is further supported by the effect of captopril, an ACE inhibitor, in inducing accelerated involution of IH. The discovery of the critical role of RAS in IH represents a novel and fascinating paradigm shift in the understanding of human development, IH, and other tumors in general.
Keywords: angiotensin-converting enzyme inhibitor; beta-blocker; captopril; hemogenic endothelium; infantile hemangioma; placenta; propranolol; renin–angiotensin system.
Figures





References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

