MicroRNA miR-125a-3p modulates molecular pathway of motility and migration in prostate cancer cells
- PMID: 25594017
- PMCID: PMC4278297
- DOI: 10.18632/oncoscience.30
MicroRNA miR-125a-3p modulates molecular pathway of motility and migration in prostate cancer cells
Abstract
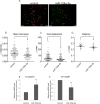
Fyn kinase is implicated in prostate cancer. We illustrate the role of miR-125a-3p in cellular pathways accounted for motility and migration of prostate cancer cells, probably through its regulation on Fyn expression and Fyn-downstream proteins. Prostate cancer PC3 cells were transiently transfected with empty miR-Vec (control) or with miR-125a-3p. Overexpression of miR-125a-3p reduced migration of PC3 cells and increased apoptosis. Live cell confocal imaging indicated that overexpression of miR-125a-3p reduced the cells' track speed and length and impaired phenotype. Fyn, FAK and paxillin, displayed reduced activity following miR-125a-3p overexpression. Accordingly, actin rearrangement and cells' protrusion formation were impaired. An inverse correlation between miR-125a-3p and Gleason score was observed in human prostate cancer tissues. Our study demonstrated that miR-125a-3p may regulate migration of prostate cancer cells.
Keywords: EMT; Fyn; actin cytoskeleton; live imaging; miR-125a-3p; migration; prostate cancer.
Conflict of interest statement
The authors declare that there is no conflict of interest that would affect the impartiality of this scientific work.
Figures





Comment in
-
miR-125a--does the difference lie in the isoform?Cell Cycle. 2015;14(6):785-6. doi: 10.1080/15384101.2015.1010961. Cell Cycle. 2015. PMID: 25790088 Free PMC article. No abstract available.
References
-
- Parsons SJ, Parsons JT. Src family kinases, key regulators of signal transduction. Oncogene. 2004;23(48):7906–7909. - PubMed
-
- Thomas SM, Brugge JS. Cellular functions regulated by Src family kinases. Annu Rev Cell Dev Biol. 1997;13:513–609. - PubMed
-
- Tang X, Feng Y, Ye K. Src-family tyrosine kinase fyn phosphorylates phosphatidylinositol 3-kinase enhancer-activating Akt, preventing its apoptotic cleavage and promoting cell survival. Cell Death Differ. 2007;14(2):368–377. - PubMed
-
- Prag S, Lepekhin EA, Kolkova K, Hartmann-Petersen R, Kawa A, Walmod PS, Belman V, Gallagher HC, Berezin V, Bock E, Pedersen N. NCAM regulates cell motility. J Cell Sci. 2002;115:283–292. Pt 2. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

