Pathways driving the endocytosis of mutant and wild-type EGFR in cancer
- PMID: 25594057
- PMCID: PMC4278327
- DOI: 10.18632/oncoscience.67
Pathways driving the endocytosis of mutant and wild-type EGFR in cancer
Abstract
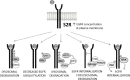
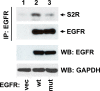
EGFR (epidermal growth factor receptor) is activated through changes in expression or mutations in a number of tumors and is a driving force in cancer progression. EGFR is targeted by numerous inhibitors, including chimeric antibodies targeting the extracellular domain and small molecule kinase domain inhibitors. The kinase domain inhibitors are particularly active against mutant forms of the receptor, and subsequent mutations drive resistance to the inhibitors. Here, we review recent developments on the trafficking of wild-type and mutant EGFR, focusing on the roles of MIG6, SPRY2, ITSN, SHP2, S2R(PGRMC1) and RAK. Some classes of EGFR regulators affect wild-type and mutant EGFR equally, while others are specific for either the wild-type or mutant form of the receptor. Below we summarize multiple signaling-associated pathways that are important in trafficking wild-type and mutant EGFR with the goal being stimulation of new approaches for targeting the distinct forms of the receptor.
Keywords: endocytosis; kinase; receptor; signaling; therapeutics.
Figures
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

