Predictive motor control of sensory dynamics in auditory active sensing
- PMID: 25594376
- PMCID: PMC4898262
- DOI: 10.1016/j.conb.2014.12.005
Predictive motor control of sensory dynamics in auditory active sensing
Abstract
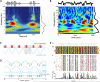
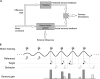
Neuronal oscillations present potential physiological substrates for brain operations that require temporal prediction. We review this idea in the context of auditory perception. Using speech as an exemplar, we illustrate how hierarchically organized oscillations can be used to parse and encode complex input streams. We then consider the motor system as a major source of rhythms (temporal priors) in auditory processing, that act in concert with attention to sharpen sensory representations and link them across areas. We discuss the circuits that could mediate this audio-motor interaction, notably the potential role of the somatosensory system. Finally, we reposition temporal predictions in the context of internal models, discussing how they interact with feature-based or spatial predictions. We argue that complementary predictions interact synergistically according to the organizational principles of each sensory system, forming multidimensional filters crucial to perception.
Copyright © 2015. Published by Elsevier Ltd.
Figures


References
-
- von Helmholtz H. Handbook of physiological optics. Leipzig: Leopold Voss. 1856 no volume.
-
- Sadaghiani S, Kleinschmidt A. Functional interactions between intrinsic brain activity and behavior. NeuroImage. 2013;80:379–386. - PubMed
-
- Arnal L, Giraud AL. Cortical oscillations and sensory predictions. Trends in Cognitive Sciences. 2012;16:390–398. This review paper reconciles predictive coding and neuronal oscillations frameworks. It specifically describes how what and when predictions could be computationally implemented by distinct neuronal rhythms, and addresses the causal role of the motor system in temporal predictions. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

