Protein lysine acetylation by p300/CBP
- PMID: 25594381
- PMCID: PMC4378506
- DOI: 10.1021/cr500452k
Protein lysine acetylation by p300/CBP
Erratum in
-
Correction to Protein Lysine Acetylation by p300/CBP.Chem Rev. 2016 Jul 27;116(14):8314. doi: 10.1021/acs.chemrev.6b00351. Epub 2016 Jun 15. Chem Rev. 2016. PMID: 27304234 Free PMC article. No abstract available.
Figures







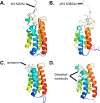



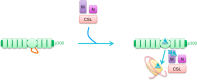

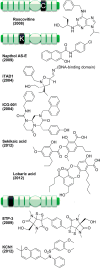

References
-
- Waddington C. H. Endeavour 1942, 18.
-
- Klar A. J. Trends Genet. 1998, 14, 299. - PubMed
-
- Redon C.; Pilch D.; Rogakou E.; Sedelnikova O.; Newrock K.; Bonner W. Curr. Opin. Genet. Dev. 2002, 12, 162. - PubMed
-
- Dawson M. A.; Kouzarides T. Cell 2012, 150, 12. - PubMed
-
- Mukherjee S.; Hao Y. H.; Orth K. Trends Biochem. Sci. 2007, 32, 210. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

