Increased sleep need and daytime sleepiness 6 months after traumatic brain injury: a prospective controlled clinical trial
- PMID: 25595147
- PMCID: PMC4408434
- DOI: 10.1093/brain/awu391
Increased sleep need and daytime sleepiness 6 months after traumatic brain injury: a prospective controlled clinical trial
Abstract
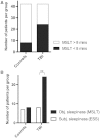
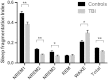
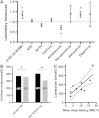
Post-traumatic sleep-wake disturbances are common after acute traumatic brain injury. Increased sleep need per 24 h and excessive daytime sleepiness are among the most prevalent post-traumatic sleep disorders and impair quality of life of trauma patients. Nevertheless, the relation between traumatic brain injury and sleep outcome, but also the link between post-traumatic sleep problems and clinical measures in the acute phase after traumatic brain injury has so far not been addressed in a controlled and prospective approach. We therefore performed a prospective controlled clinical study to examine (i) sleep-wake outcome after traumatic brain injury; and (ii) to screen for clinical and laboratory predictors of poor sleep-wake outcome after acute traumatic brain injury. Forty-two of 60 included patients with first-ever traumatic brain injury were available for follow-up examinations. Six months after trauma, the average sleep need per 24 h as assessed by actigraphy was markedly increased in patients as compared to controls (8.3 ± 1.1 h versus 7.1 ± 0.8 h, P < 0.0001). Objective daytime sleepiness was found in 57% of trauma patients and 19% of healthy subjects, and the average sleep latency in patients was reduced to 8.7 ± 4.6 min (12.1 ± 4.7 min in controls, P = 0.0009). Patients, but not controls, markedly underestimated both excessive sleep need and excessive daytime sleepiness when assessed only by subjective means, emphasizing the unreliability of self-assessment of increased sleep propensity in traumatic brain injury patients. At polysomnography, slow wave sleep after traumatic brain injury was more consolidated. The most important risk factor for developing increased sleep need after traumatic brain injury was the presence of an intracranial haemorrhage. In conclusion, we provide controlled and objective evidence for a direct relation between sleep-wake disturbances and traumatic brain injury, and for clinically significant underestimation of post-traumatic sleep-wake disturbances by trauma patients.
Keywords: post-traumatic daytime sleepiness; post-traumatic pleiosomnia; sleep; traumatic brain injury.
© The Author (2015). Published by Oxford University Press on behalf of the Guarantors of Brain. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures


References
-
- American Academy of Sleep Medicine. . 2nd edn. Westchester, IL: American Academy of Sleep Disorders Association; 2005. International Classification of sleep disorders (ICSD-2), diagnostic and coding manual, 2nd edn.
-
- Baumann CR, Werth E, Stocker R, Ludwig S, Bassetti CL. Sleep-wake disturbances 6 months after traumatic brain injury: a prospective study. Brain. 2007;130:1873–83. - PubMed
-
- Berger RP, Pierce MC, Wisniewski SR, Adelson PD, Clark RS, Ruppel RA, et al. Neuron-specific enolase and S100B in cerebrospinal fluid after severe traumatic brain injury in infants and children. Pediatrics. 2002;109:E31. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

