Erythropoietin in amyotrophic lateral sclerosis: a multicentre, randomised, double blind, placebo controlled, phase III study
- PMID: 25595151
- PMCID: PMC4515982
- DOI: 10.1136/jnnp-2014-308996
Erythropoietin in amyotrophic lateral sclerosis: a multicentre, randomised, double blind, placebo controlled, phase III study
Abstract
Objective: To assess the efficacy of recombinant human erythropoietin (rhEPO) in amyotrophic lateral sclerosis (ALS).
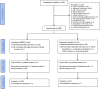
Methods: Patients with probable laboratory-supported, probable or definite ALS were enrolled by 25 Italian centres and randomly assigned (1:1) to receive intravenous rhEPO 40,000 IU or placebo fortnightly as add-on treatment to riluzole 100 mg daily for 12 months. The primary composite outcome was survival, tracheotomy or >23 h non-invasive ventilation (NIV). Secondary outcomes were ALSFRS-R, slow vital capacity (sVC) and quality of life (ALSAQ-40) decline. Tolerability was evaluated analysing adverse events (AEs) causing withdrawal. The randomisation sequence was computer-generated by blocks, stratified by centre, disease severity (ALSFRS-R cut-off score of 33) and onset (spinal or bulbar). The main outcome analysis was performed in all randomised patients and by intention-to-treat for the entire population and patients stratified by severity and onset. The study is registered, EudraCT 2009-016066-91.
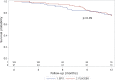
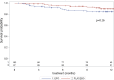
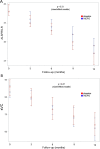
Results: We randomly assigned 208 patients, of whom 5 (1 rhEPO and 4 placebo) withdrew consent and 3 (placebo) became ineligible (retinal thrombosis, respiratory insufficiency, SOD1 mutation) before receiving treatment; 103 receiving rhEPO and 97 placebo were eligible for analysis. At 12 months, the annualised rate of death (rhEPO 0.11, 95% CI 0.06 to 0.20; placebo: 0.08, CI 0.04 to 0.17), tracheotomy or >23 h NIV (rhEPO 0.16, CI 0.10 to 0.27; placebo 0.18, CI 0.11 to 0.30) did not differ between groups, also after stratification by onset and ALSFRS-R at baseline. Withdrawal due to AE was 16.5% in rhEPO and 8.3% in placebo. No differences were found for secondary outcomes.
Conclusions: RhEPO 40,000 IU fortnightly did not change the course of ALS.
Keywords: ALS; MOTOR NEURON DISEASE.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://group.bmj.com/group/rights-licensing/permissions.
Figures

References
-
- Solling C. Organ-protective and immunomodulatory effects of erythropoietin—an update on recent clinical trials. Basic Clin Pharmacol Toxicol 2012;110:113–21. - PubMed
-
- Leist M, Ghezzi P, Grasso G, et al. Derivatives of erythropoietin that are tissue protective but not erythropoietic. Science 2004;305:239–42. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
