Mitochondrially targeted Endonuclease III has a powerful anti-infarct effect in an in vivo rat model of myocardial ischemia/reperfusion
- PMID: 25595210
- PMCID: PMC4718710
- DOI: 10.1007/s00395-014-0459-0
Mitochondrially targeted Endonuclease III has a powerful anti-infarct effect in an in vivo rat model of myocardial ischemia/reperfusion
Abstract
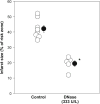
Recent reports indicate that elevating DNA glycosylase/AP lyase repair enzyme activity offers marked cytoprotection in cultured cells and a variety of injury models. In this study, we measured the effect of EndoIII, a fusion protein construct that traffics Endonuclease III, a DNA glycosylase/AP lyase, to the mitochondria, on infarct size in a rat model of myocardial ischemia/reperfusion. Open-chest, anesthetized rats were subjected to 30 min of occlusion of a coronary artery followed by 2 h of reperfusion. An intravenous bolus of EndoIII, 8 mg/kg, just prior to reperfusion reduced infarct size from 43.8 ± 1.4% of the risk zone in control animals to 24.0 ± 1.3% with no detectable hemodynamic effect. Neither EndoIII's vehicle nor an enzymatically inactive EndoIII mutant (K120Q) offered any protection. The magnitude of EndoIII's protection was comparable to that seen with the platelet aggregation inhibitor cangrelor (25.0 ± 1.8% infarction of risk zone). Because loading with a P2Y12 receptor blocker to inhibit platelets is currently the standard of care for treatment of acute myocardial infarction, we tested whether EndoIII could further reduce infarct size in rats treated with a maximally protective dose of cangrelor. The combination reduced infarct size to 15.1 ± 0.9% which was significantly smaller than that seen with either cangrelor or EndoIII alone. Protection from cangrelor but not EndoIII was abrogated by pharmacologic blockade of phosphatidylinositol-3 kinase or adenosine receptors indicating differing cellular mechanisms. We hypothesized that EndoIII protected the heart from spreading necrosis by preventing the release of proinflammatory fragments of mitochondrial DNA (mtDNA) into the heart tissue. In support of this hypothesis, an intravenous bolus at reperfusion of deoxyribonuclease I (DNase I) which should degrade any DNA fragments escaping into the extracellular space was as protective as EndoIII. Furthermore, the combination of EndoIII and DNase I produced additive protection. While EndoIII would maintain mitochondrial integrity in many of the ischemic cardiomyocytes, DNase I would further prevent mtDNA released from those cells that EndoIII could not save from propagating further necrosis. Thus, our mtDNA hypothesis would predict additive protection. Finally to demonstrate the toxicity of mtDNA, isolated hearts were subjected to 15 min of global ischemia. Infarct size doubled when the coronary vasculature was filled with mtDNA fragments during the period of global ischemia. To our knowledge, EndoIII and DNase are the first agents that can both be given at reperfusion and add to the protection of a P2Y12 blocker, and thus should be effective in today's patient with acute myocardial infarction.
Conflict of interest statement
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

