Molecular basis of embryonic stem cell self-renewal: from signaling pathways to pluripotency network
- PMID: 25595304
- PMCID: PMC4809369
- DOI: 10.1007/s00018-015-1833-2
Molecular basis of embryonic stem cell self-renewal: from signaling pathways to pluripotency network
Abstract
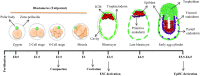
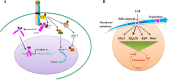
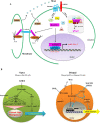
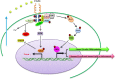
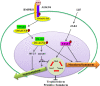
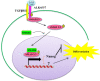
Embryonic stem cells (ESCs) can be maintained in culture indefinitely while retaining the capacity to generate any type of cell in the body, and therefore not only hold great promise for tissue repair and regeneration, but also provide a powerful tool for modeling human disease and understanding biological development. In order to fulfill the full potential of ESCs, it is critical to understand how ESC fate, whether to self-renew or to differentiate into specialized cells, is regulated. On the molecular level, ESC fate is controlled by the intracellular transcriptional regulatory networks that respond to various extrinsic signaling stimuli. In this review, we discuss and compare important signaling pathways in the self-renewal and differentiation of mouse, rat, and human ESCs with an emphasis on how these pathways integrate into ESC-specific transcription circuitries. This will be beneficial for understanding the common and conserved mechanisms that govern self-renewal, and for developing novel culture conditions that support ESC derivation and maintenance.
Figures
Similar articles
-
Novel insights into embryonic stem cell self-renewal revealed through comparative human and mouse systems biology networks.Stem Cells. 2014 May;32(5):1161-72. doi: 10.1002/stem.1612. Stem Cells. 2014. PMID: 24307629 Free PMC article.
-
Evolutionarily conserved transcriptional co-expression guiding embryonic stem cell differentiation.PLoS One. 2008;3(10):e3406. doi: 10.1371/journal.pone.0003406. Epub 2008 Oct 15. PLoS One. 2008. PMID: 18923680 Free PMC article.
-
Retinoic acid maintains self-renewal of murine embryonic stem cells via a feedback mechanism.Differentiation. 2008 Nov;76(9):931-45. doi: 10.1111/j.1432-0436.2008.00272.x. Epub 2008 Jul 1. Differentiation. 2008. PMID: 18637026
-
The role of Ca(2+) signaling on the self-renewal and neural differentiation of embryonic stem cells (ESCs).Cell Calcium. 2016 Mar;59(2-3):67-74. doi: 10.1016/j.ceca.2016.01.004. Epub 2016 Feb 27. Cell Calcium. 2016. PMID: 26973143 Review.
-
Regulatory role of Klf5 in early mouse development and in embryonic stem cells.Vitam Horm. 2011;87:381-97. doi: 10.1016/B978-0-12-386015-6.00037-8. Vitam Horm. 2011. PMID: 22127252 Review.
Cited by
-
LIF signal in mouse embryonic stem cells.JAKSTAT. 2015 Sep 11;4(2):e1086520. doi: 10.1080/21623996.2015.1086520. eCollection 2015. JAKSTAT. 2015. PMID: 27127728 Free PMC article. Review.
-
Research progress and therapeutic prospect of PHF5A acting as a new target for malignant tumors.Zhejiang Da Xue Xue Bao Yi Xue Ban. 2022 Nov 25;51(5):647-655. doi: 10.3724/zdxbyxb-2022-0459. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2022. PMID: 36581580 Free PMC article. Review. English.
-
Mouse Embryonic Stem Cell Culture in Serum-Containing or 2i Conditions.Methods Mol Biol. 2022;2520:275-294. doi: 10.1007/7651_2021_438. Methods Mol Biol. 2022. PMID: 34661879
-
Opportunities lost and gained: Changes in progenitor competence during nervous system development.Neurogenesis (Austin). 2017 May 26;4(1):e1324260. doi: 10.1080/23262133.2017.1324260. eCollection 2017. Neurogenesis (Austin). 2017. PMID: 28656157 Free PMC article. Review.
-
Embryonic stem cell-derived extracellular vesicles rejuvenate senescent cells and antagonize aging in mice.Bioact Mater. 2023 Jul 1;29:85-97. doi: 10.1016/j.bioactmat.2023.06.011. eCollection 2023 Nov. Bioact Mater. 2023. PMID: 37449253 Free PMC article.
References
-
- Smith AG. Embryo-derived stem cells: of mice and men. Annu Rev Cell Dev Biol. 2001;17:435–462. - PubMed
-
- Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–156. - PubMed
-
- Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. - PubMed
-
- Buehr M, Meek S, Blair K, Yang J, Ure J, Silva J, et al. Capture of authentic embryonic stem cells from rat blastocysts. Cell. 2008;135:1287–1298. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

