Hypotonic stress induces RANKL via transient receptor potential melastatin 3 (TRPM3) and vaniloid 4 (TRPV4) in human PDL cells
- PMID: 25595364
- PMCID: PMC4814022
- DOI: 10.1177/0022034514567196
Hypotonic stress induces RANKL via transient receptor potential melastatin 3 (TRPM3) and vaniloid 4 (TRPV4) in human PDL cells
Abstract
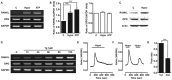
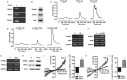
Bone remodeling occurs in response to various types of mechanical stress. The periodontal ligament (PDL) plays an important role in mechanical stress-mediated alveolar bone remodeling. However, the underlying mechanism at the cellular level has not been extensively studied. In this study, we investigated the effect of shear stress on the expression of bone remodeling factors, including receptor activator of nuclear factor-kappa B (NF-κB) ligand (RANKL) and osteoprotegerin (OPG), as well as its upstream signaling pathway in primary human PDL cells. We applied hypotonic stress to reproduce shear stress to PDL cells. Hypotonic stress induced the messenger RNA (mRNA) and protein expression of RANKL but not OPG. It also increased intracellular Ca(2+) concentration ([Ca(2+)]i). Extracellular Ca(2+) depletion and nonspecific plasma membrane Ca(2+) channel blockers completely inhibited the increase in both [Ca(2+)]i and RANKL mRNA expression. We identified the expression and activation of transient receptor potential melastatin 3 (TRPM3) and vaniloid 4 (TRPV4) channels in PDL cells. Pregnenolone sulfate (PS) and 4α-phorbol 12, 13-didecanoate (4α-PDD), which are agonists of TRPM3 and TRPV4, augmented Ca(2+) influx and RANKL mRNA expression. Both pharmacological (2-aminoethoxydiphenyl borate [2-APB], ruthenium red [RR], ononetin [Ono], and HC 067047 [HC]) and genetic (small interfering RNA [siRNA]) inhibitors of TRPM3 and TRPV4 reduced the hypotonic stress-mediated increase in [Ca(2+)]i and RANKL mRNA expression. Our study shows that hypotonic stress induced RANKL mRNA expression via TRPM3- and TRPV4-mediated extracellular Ca(2+) influx and RANKL expression. This signaling pathway in PDL cells may play a critical role in mechanical stress-mediated alveolar bone remodeling.
Keywords: bone remodeling/regeneration; cell signaling; ion channels; mechanotransduction; osmotic stress; periodontal ligament.
© International & American Associations for Dental Research 2015.
Conflict of interest statement
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
Figures



Similar articles
-
Compressive stress induced the up-regulation of M-CSF, RANKL, TNF-α expression and the down-regulation of OPG expression in PDL cells via the integrin-FAK pathway.Arch Oral Biol. 2013 Jun;58(6):707-16. doi: 10.1016/j.archoralbio.2012.11.003. Epub 2012 Dec 4. Arch Oral Biol. 2013. PMID: 23219295
-
TRPM3/TRPV4 regulates Ca2+-mediated RANKL/NFATc1 expression in osteoblasts.J Mol Endocrinol. 2018 Oct 15;61(4):207-218. doi: 10.1530/JME-18-0051. J Mol Endocrinol. 2018. PMID: 30328352
-
Functional analysis of core binding factor a1 and its relationship with related genes expressed by human periodontal ligament cells exposed to mechanical stress.Eur J Orthod. 2010 Dec;32(6):698-705. doi: 10.1093/ejo/cjq010. Epub 2010 Jun 4. Eur J Orthod. 2010. PMID: 20525800
-
RANK/RANKL/OPG during orthodontic tooth movement.Orthod Craniofac Res. 2009 May;12(2):113-9. doi: 10.1111/j.1601-6343.2009.01444.x. Orthod Craniofac Res. 2009. PMID: 19419454 Review.
-
Transient receptor potential TRPM3 channels: Pharmacology, signaling, and biological functions.Pharmacol Res. 2017 Oct;124:92-99. doi: 10.1016/j.phrs.2017.07.014. Epub 2017 Jul 16. Pharmacol Res. 2017. PMID: 28720517 Review.
Cited by
-
TRPM3_miR-204: a complex locus for eye development and disease.Hum Genomics. 2020 Feb 18;14(1):7. doi: 10.1186/s40246-020-00258-4. Hum Genomics. 2020. PMID: 32070426 Free PMC article. Review.
-
Effect of loaded orthodontic miniscrew implant on compressive stresses in adjacent periodontal ligament.Angle Orthod. 2019 Mar;89(2):235-241. doi: 10.2319/122017-873.1. Epub 2018 Sep 19. Angle Orthod. 2019. PMID: 30230377 Free PMC article.
-
Neural signalling of gut mechanosensation in ingestive and digestive processes.Nat Rev Neurosci. 2022 Mar;23(3):135-156. doi: 10.1038/s41583-021-00544-7. Epub 2022 Jan 4. Nat Rev Neurosci. 2022. PMID: 34983992 Review.
-
TRPC expression in human periodontal ligament cells and the periodontal tissue of periodontitis mice: a preliminary study.Lab Anim Res. 2023 Sep 1;39(1):19. doi: 10.1186/s42826-023-00171-6. Lab Anim Res. 2023. PMID: 37653550 Free PMC article.
-
The roles of mechanosensitive ion channels and associated downstream MAPK signaling pathways in PDLC mechanotransduction.Mol Med Rep. 2020 May;21(5):2113-2122. doi: 10.3892/mmr.2020.11006. Epub 2020 Feb 27. Mol Med Rep. 2020. PMID: 32323761 Free PMC article.
References
-
- Basdra EK, Komposch G. 1997. Osteoblast-like properties of human periodontal ligament cells: an in vitro analysis. Eur J Orthod. 19(6):615–621. - PubMed
-
- Bergomi M, Cugnoni J, Botsis J, Belser UC, Anselm Wiskott HW. 2010. The role of the fluid phase in the viscous response of bovine periodontal ligament. J Biomech. 43(6):1146–1152. - PubMed
-
- Boyle WJ, Simonet WS, Lacey DL. 2003. Osteoclast differentiation and activation. Nature. 423(6937):337–342. - PubMed
-
- Everaerts W, Zhen X, Ghosh D, Vriens J, Gevaert T, Gilbert JP, Hayward NJ, McNamara CR, Xue F, Moran MM, et al. 2010. Inhibition of the cation channel TRPV4 improves bladder function in mice and rats with cyclophosphamide-induced cystitis. Proc Natl Acad Sci U S A. 107(44):19084–19089. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

