Wntless regulates dentin apposition and root elongation in the mandibular molar
- PMID: 25595365
- PMCID: PMC4814015
- DOI: 10.1177/0022034514567198
Wntless regulates dentin apposition and root elongation in the mandibular molar
Abstract
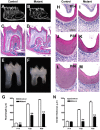
Wnt signaling plays an essential role in the dental epithelium and mesenchyme during tooth morphogenesis. However, it remains unclear if Wnt ligands, produced from dental mesenchyme, are necessary for odontoblast differentiation and dentin formation. Here, we show that odontoblast-specific disruption of Wntless (Wls), a chaperon protein that regulates Wnt sorting and secretion, leads to severe defects in dentin formation and root elongation. Dentin thickness decreased remarkably and pulp chambers enlarged in the mandibular molars of OC-Cre;Wls(CO/CO) mice. Although the initial odontoblast differentiation was normal in the mutant crown, odontoblasts became cuboidal and dentin thickness was reduced. In immunohistochemistry, Wnt10a, β-catenin, type I collagen, and dentin sialoprotein were significantly down-regulated in the odontoblasts of mutant crown. In addition, roots were short and root canals were widened. Cell proliferation was reduced in the developing root apex of mutant molars. Furthermore, Wnt10a and Axin2 expression was remarkably decreased in the odontoblasts of mutant roots. Deletion of the Wls gene in odontoblasts appears to reduce canonical Wnt activity, leading to inhibition of odontoblast maturation and root elongation.
Keywords: Wnt signaling pathway; dentinogenesis; mice; odontoblasts; tooth development; tooth roots.
© International & American Associations for Dental Research 2015.
Conflict of interest statement
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
Figures



References
-
- Banziger C, Soldini D, Schütt C, Zipperlen P, Hausmann G, Basler K. 2006. Wntless, a conserved membrane protein dedicated to the secretion of Wnt proteins from signaling cells. Cell. 125(3):509–522. - PubMed
-
- Bartscherer K, Pelte N, Ingelfinger D, Boutros M. 2006. Secretion of Wnt ligands requires Evi, a conserved transmembrane protein. Cell. 125(3):523–533. - PubMed
-
- Fjeld K, Kettunen P, Furmanek T, Kvinnsland IH, Luukko K. 2005. Dynamic expression of Wnt signaling-related Dickkopf1, -2, and -3 mRNAs in the developing mouse tooth. Dev Dyn. 233(1):161–166. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

