Hepatotoxicity from antituberculous therapy in the elderly: a systematic review
- PMID: 25595441
- PMCID: PMC4640443
- DOI: 10.1016/j.tube.2014.10.006
Hepatotoxicity from antituberculous therapy in the elderly: a systematic review
Abstract
Background: Elderly persons have the highest rates of tuberculosis (TB) in the United States compared to all other age groups. A systematic literature review was conducted to determine if older age was a risk factor for hepatotoxicity resulting from treatment with first-line drugs used to treat active (TB) and latent tuberculosis (LTBI).
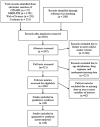
Methods: A systematic review of MEDLINE, Cochrane Controlled Trial Registry, CINAHL(®), and Science Citation Index Expanded (from 1970 to 2011) was performed to determine the risk of hepatotoxicity, comparing those over 60 with those under 60. A meta-analysis was performed using a random effects model along with log odds ratios and the chi-square test.
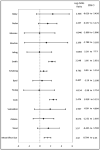
Findings: Thirty-eight studies (40,034 participants; 1208 cases of hepatotoxicity) met the selection criteria. For active TB, an overall mean effect of 0.277 (p = 0.024, 95% CI: 0.037-0.517) was observed, which is equivalent to an odds ratio of 1.32 (95% CI: 1.04-1.68). For LTBI, an overall mean effect of 1.42 (p < 0.001, 95% CI: 0.794-2.05) was observed, which translates to an odds ratio of 4.14 (95% CI: 2.21-7.74).
Interpretation: Our analysis revealed that patients older than 60 had significantly more risk of hepatotoxicity. These studies suggest that a gentler regimen of treatment for older individuals could benefit health outcomes in this population of TB patients and minimize risks to the public's health.
Keywords: Aging; Antituberculous; Elderly; Hepatotoxicity; Latent tuberculosis; Systematic review; Therapy; Toxicity; Treatment; Tuberculosis.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Figures

References
-
- Centers for Disease Control and Prevention (CDC) Reported tuberculosis in the United States, 2011. Atlanta, GA: U.S. Department of Health and Human Services; 2012.
-
- Devarbhavi H. Antituberculous drug-induced liver injury: current perspective. Trop Gastroenterol. 2011;32(3):167–74. - PubMed
-
- Saukkonen JJ. An official ATS statement: hepatotoxicity of antituberculosis therapy. Am J Respir Crit Care Med. 2006;174(8):935. - PubMed
-
- Blumberg HM. American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America: treatment of tuberculosis. Am J Respir Crit Care Med. 2003;167(4):603–62. - PubMed
-
- Griffith DE. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175(4):367. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

