Elizabethkingia anophelis: molecular manipulation and interactions with mosquito hosts
- PMID: 25595771
- PMCID: PMC4345385
- DOI: 10.1128/AEM.03733-14
Elizabethkingia anophelis: molecular manipulation and interactions with mosquito hosts
Abstract
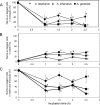
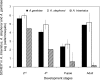
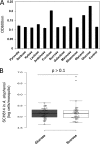
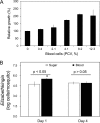
Flavobacteria (members of the family Flavobacteriaceae) dominate the bacterial community in the Anopheles mosquito midgut. One such commensal, Elizabethkingia anophelis, is closely associated with Anopheles mosquitoes through transstadial persistence (i.e., from one life stage to the next); these and other properties favor its development for paratransgenic applications in control of malaria parasite transmission. However, the physiological requirements of E. anophelis have not been investigated, nor has its capacity to perpetuate despite digestion pressure in the gut been quantified. To this end, we first developed techniques for genetic manipulation of E. anophelis, including selectable markers, reporter systems (green fluorescent protein [GFP] and NanoLuc), and transposons that function in E. anophelis. A flavobacterial expression system based on the promoter PompA was integrated into the E. anophelis chromosome and showed strong promoter activity to drive GFP and NanoLuc reporter production. Introduced, GFP-tagged E. anophelis associated with mosquitoes at successive developmental stages and propagated in Anopheles gambiae and Anopheles stephensi but not in Aedes triseriatus mosquitoes. Feeding NanoLuc-tagged cells to A. gambiae and A. stephensi in the larval stage led to infection rates of 71% and 82%, respectively. In contrast, a very low infection rate (3%) was detected in Aedes triseriatus mosquitoes under the same conditions. Of the initial E. anophelis cells provided to larvae, 23%, 71%, and 85% were digested in A. stephensi, A. gambiae, and Aedes triseriatus, respectively, demonstrating that E. anophelis adapted to various mosquito midgut environments differently. Bacterial cell growth increased up to 3-fold when arginine was supplemented in the defined medium. Furthermore, the number of NanoLuc-tagged cells in A. stephensi significantly increased when arginine was added to a sugar diet, showing it to be an important amino acid for E. anophelis. Animal erythrocytes promoted E. anophelis growth in vivo and in vitro, indicating that this bacterium could obtain nutrients by participating in erythrocyte lysis in the mosquito midgut.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures


References
-
- Boissière A, Tchioffo MT, Bachar D, Abate L, Marie A, Nsango SE, Shahbazkia HR, Awono-Ambene PH, Levashina EA, Christen R, Morlais I. 2012. Midgut microbiota of the malaria mosquito vector Anopheles gambiae and interactions with Plasmodium falciparum infection. PLoS Pathog 8:e1002742. doi:10.1371/journal.ppat.1002742. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

