Lipid peroxidation in brain or spinal cord mitochondria after injury
- PMID: 25595872
- PMCID: PMC4506732
- DOI: 10.1007/s10863-015-9600-5
Lipid peroxidation in brain or spinal cord mitochondria after injury
Abstract
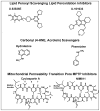
Extensive evidence has demonstrated an important role of oxygen radical formation (i.e., oxidative stress) as a mediator of the secondary injury process that occurs following primary mechanical injury to the brain or spinal cord. The predominant form of oxygen radical-induced oxidative damage that occurs in injured nervous tissue is lipid peroxidation (LP). Much of the oxidative stress in injured nerve cells initially begins in mitochondria via the generation of the reactive nitrogen species peroxynitrite (PN) which then can generate multiple highly reactive free radicals including nitrogen dioxide (•NO2), hydroxyl radical (•OH) and carbonate radical (•CO3). Each can readily induce LP within the phospholipid membranes of the mitochondrion leading to respiratory dysfunction, calcium buffering impairment, mitochondrial permeability transition and cell death. Validation of the role of LP in central nervous system secondary injury has been provided by the mitochondrial and neuroprotective effects of multiple antioxidant agents which are briefly reviewed.
Keywords: Antioxidant; Lipid peroxidation; Mitochondria; Oxidative stress; Spinal cord injury; Traumatic brain injury.
Figures
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

