Crystal structure of human persulfide dioxygenase: structural basis of ethylmalonic encephalopathy
- PMID: 25596185
- PMCID: PMC4383860
- DOI: 10.1093/hmg/ddv007
Crystal structure of human persulfide dioxygenase: structural basis of ethylmalonic encephalopathy
Abstract
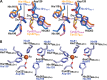
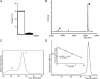
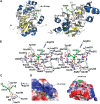
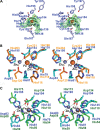
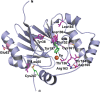
The ethylmalonic encephalopathy protein 1 (ETHE1) catalyses the oxygen-dependent oxidation of glutathione persulfide (GSSH) to give persulfite and glutathione. Mutations to the hETHE1 gene compromise sulfide metabolism leading to the genetic disease ethylmalonic encephalopathy. hETHE1 is a mono-iron binding member of the metallo-β-lactamase (MBL) fold superfamily. We report crystallographic analysis of hETHE1 in complex with iron to 2.6 Å resolution. hETHE1 contains an αββα MBL-fold, which supports metal-binding by the side chains of an aspartate and two histidine residues; three water molecules complete octahedral coordination of the iron. The iron binding hETHE1 enzyme is related to the 'classical' di-zinc binding MBL hydrolases involved in antibiotic resistance, but has distinctive features. The histidine and aspartate residues involved in iron-binding in ETHE1, occupy similar positions to those observed across both the zinc 1 and zinc 2 binding sites in classical MBLs. The active site of hETHE1 is very similar to an ETHE1-like enzyme from Arabidopsis thaliana (60% sequence identity). A channel leading to the active site is sufficiently large to accommodate a GSSH substrate. Some of the observed hETHE1 clinical mutations cluster in the active site region. The structure will serve as a basis for detailed functional and mechanistic studies on ETHE1 and will be useful in the development of selective MBL inhibitors.
© The Author 2015. Published by Oxford University Press.
Figures



References
-
- Grosso S., Mostardini R., Farnetani M.A., Molinelli M., Berardi R., Dionisi-Vici C., Rizzo C., Morgese G., Balestri P. (2002) Ethylmalonic encephalopathy: further clinical and neuroradiological characterization. J. Neurol., 249, 1446–1450. - PubMed
-
- Pigeon N., Campeau P.M., Cyr D., Lemieux B., Clarke J.T. (2009) Clinical heterogeneity in ethylmalonic encephalopathy. J. Child. Neurol., 24, 991–996. - PubMed
-
- Barth M., Ottolenghi C., Hubert L., Chrétien D., Serre V., Gobin S., Romano S., Vassault A., Sefiani A., Ricquier D., et al. (2010) Multiple sources of metabolic disturbance in ETHE1-related ethylmalonic encephalopathy. J. Inherit. Metab. Dis., 33, S443–S453. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

