The efficacy of activated protein C for the treatment of sepsis: incorporating observational evidence with a Bayesian approach
- PMID: 25596198
- PMCID: PMC4298096
- DOI: 10.1136/bmjopen-2014-006524
The efficacy of activated protein C for the treatment of sepsis: incorporating observational evidence with a Bayesian approach
Abstract
Objective: The present study aimed to combine observational evidence with randomised controlled trials (RCTs) by using the Bayesian approach.
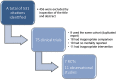
Data sources: Electronic databases, including PubMed, Cochrane Central Register of Controlled Trials (CENTRAL), ISI Web of Science, EMBASE and EBSCO were searched from inception to January 2014.
Study eligibility: RCTs and observational studies (OS) investigating the effectiveness of activated protein C (aPC) on mortality reduction were included for analysis.
Participants: Patients with sepsis.
Intervention: aPC.
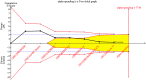
Synthesis methods: Observational evidence was incorporated into the analysis by using power transformed priors in a Bayesian. Trial sequential analysis was performed to examine changes over time and whether further studies need to be conducted.
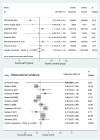
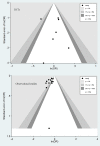
Main results: a total of 7 RCTs and 12 OS were included for the analysis. There was moderate heterogeneity among included RCTs (I(2)=48.6%, p=0.07). The pooled OR for mortality from RCTs was 1.00 (95% CI 0.84 to 1.19). In OS, there was potential publication bias as indicated by the funnel plot and the pooled OR for mortality with the use of aPC was 0.67 (95% CI 0.62 to 0.72). The pooled effect sizes of RCTs were changed by using different power transform priors derived from observational evidence. When observational evidence was used at its 'face value', the treatment effect of aPC was statistically significant in reducing mortality.
Conclusions: while RCT evidence showed no beneficial effect of aPC on sepsis, observational evidence showed a significant treatment effect of aPC. By using power transform priors in Bayesian model, we explicitly demonstrated how RCT evidence could be changed by observational evidence.
Trial registration number: The protocol for the current study was registered in PROSPERO (registration number: CRD42014009562).
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://group.bmj.com/group/rights-licensing/permissions.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
