Hormonal regulation of colour change in eyes of a cryptic fish
- PMID: 25596278
- PMCID: PMC4365489
- DOI: 10.1242/bio.20149993
Hormonal regulation of colour change in eyes of a cryptic fish
Abstract
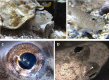
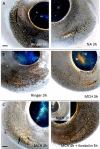
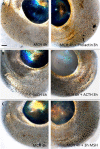
Colour change of the skin in lower vertebrates such as fish has been a subject of great scientific and public interest. However, colour change also takes place in eyes of fish and while an increasing amount of data indicates its importance in behaviour, very little is known about its regulation. Here, we report that both eye and skin coloration change in response to white to black background adaptation in live sand goby Pomatoschistus minutes, a bentic marine fish. Through in vitro experiments, we show that noradrenaline and melanocyte concentrating hormone (MCH) treatments cause aggregation of pigment organelles in the eye chromatophores. Daylight had no aggregating effect. Combining forskolin to elevate intracellular cyclic adenosine monophosphate (cAMP) with MCH resulted in complete pigment dispersal and darkening of the eyes, whereas combining prolactin, adrenocorticotrophic hormone (ACTH) or melanocyte stimulating hormone (α-MSH) with MCH resulted in more yellow and red eyes. ACTH and MSH also induced dispersal in the melanophores, resulting in overall darker eyes. By comparing analysis of eyes, skin and peritoneum, we conclude that the regulation pattern is similar between these different tissues in this species which is relevant for the cryptic life strategy of this species. With the exception of ACTH which resulted in most prominent melanophore pigment dispersal in the eyes, all other treatments provided similar results between tissue types. To our knowledge, this is the first study that has directly analysed hormonal regulation of physiological colour change in eyes of fish.
Keywords: Camouflage; Erythrophores; Iris; Melanophores; Physiological colour change; Pigment.
© 2015. Published by The Company of Biologists Ltd.
Conflict of interest statement
Figures
Similar articles
-
Regulation of eye and jaw colouration in three-spined stickleback Gasterosteus aculeatus.J Fish Biol. 2018 Jun;92(6):1788-1804. doi: 10.1111/jfb.13620. Epub 2018 Apr 25. J Fish Biol. 2018. PMID: 29577284
-
Taisho-Sanshoku koi have hardly faded skin and show attenuated melanophore sensitivity to adrenaline and melanin-concentrating hormone.Front Endocrinol (Lausanne). 2022 Dec 22;13:994060. doi: 10.3389/fendo.2022.994060. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 36619537 Free PMC article.
-
Inhibiting roles of melanin-concentrating hormone for skin pigment dispersion in barfin flounder, Verasper moseri.Gen Comp Endocrinol. 2011 Mar 1;171(1):75-81. doi: 10.1016/j.ygcen.2010.12.008. Epub 2010 Dec 23. Gen Comp Endocrinol. 2011. PMID: 21185295
-
Interrelation between melanocyte-stimulating hormone and melanin-concentrating hormone in physiological body color change: roles emerging from barfin flounder Verasper moseri.Gen Comp Endocrinol. 2013 Jan 15;181:229-34. doi: 10.1016/j.ygcen.2012.09.026. Epub 2012 Nov 17. Gen Comp Endocrinol. 2013. PMID: 23168086 Review.
-
Fish Chromatophores--From Molecular Motors to Animal Behavior.Int Rev Cell Mol Biol. 2016;321:171-219. doi: 10.1016/bs.ircmb.2015.09.005. Epub 2015 Oct 31. Int Rev Cell Mol Biol. 2016. PMID: 26811288 Review.
Cited by
-
Dark eyes in female sand gobies indicate readiness to spawn.PLoS One. 2017 Jun 7;12(6):e0177714. doi: 10.1371/journal.pone.0177714. eCollection 2017. PLoS One. 2017. PMID: 28591156 Free PMC article.
-
Asymmetry of eye color in the common cuckoo.Sci Rep. 2017 Aug 8;7(1):7612. doi: 10.1038/s41598-017-08071-1. Sci Rep. 2017. PMID: 28790375 Free PMC article.
-
Neurotransmitter norepinephrine regulates chromatosomes aggregation and the formation of blotches in coral trout Plectropomus leopardus.Fish Physiol Biochem. 2024 Apr;50(2):705-719. doi: 10.1007/s10695-024-01300-1. Epub 2024 Jan 31. Fish Physiol Biochem. 2024. PMID: 38294642
-
Ultrastructure and regulation of color change in blue spots of leopard coral trout Plectropomus leopardus.Front Endocrinol (Lausanne). 2022 Oct 20;13:984081. doi: 10.3389/fendo.2022.984081. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 36339398 Free PMC article.
-
A novel mutation of the StAR gene with congenital adrenal hyperplasia and its association with heterochromia iridis: a case report.BMC Endocr Disord. 2019 Oct 30;19(1):116. doi: 10.1186/s12902-019-0448-2. BMC Endocr Disord. 2019. PMID: 31666050 Free PMC article.
References
-
- Forsgren E. (1999). Sexual selection and sex roles in the sand goby. Behaviour and Conservation of Littoral Fishes Almada V. C., Oliveira R. F., Gonçalves E. J., 249–274.Lisboa: ISPA.
-
- Frost P. (2006). European hair and eye colour: a case of frequency-dependent sexual selection? Evol. Hum. Behav. 27, 85–103. 10.1016/j.evolhumbehav.2005.07.002 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

