Conditional knock-out reveals a requirement for O-linked N-Acetylglucosaminase (O-GlcNAcase) in metabolic homeostasis
- PMID: 25596529
- PMCID: PMC4358131
- DOI: 10.1074/jbc.M114.617779
Conditional knock-out reveals a requirement for O-linked N-Acetylglucosaminase (O-GlcNAcase) in metabolic homeostasis
Abstract
O-GlcNAc cycling is maintained by the reciprocal activities of the O-GlcNAc transferase and the O-GlcNAcase (OGA) enzymes. O-GlcNAc transferase is responsible for O-GlcNAc addition to serine and threonine (Ser/Thr) residues and OGA for its removal. Although the Oga gene (MGEA5) is a documented human diabetes susceptibility locus, its role in maintaining insulin-glucose homeostasis is unclear. Here, we report a conditional disruption of the Oga gene in the mouse. The resulting homozygous Oga null (KO) animals lack OGA enzymatic activity and exhibit elevated levels of the O-GlcNAc modification. The Oga KO animals showed nearly complete perinatal lethality associated with low circulating glucose and low liver glycogen stores. Defective insulin-responsive GSK3β phosphorylation was observed in both heterozygous (HET) and KO Oga animals. Although Oga HET animals were viable, they exhibited alterations in both transcription and metabolism. Transcriptome analysis using mouse embryonic fibroblasts revealed deregulation in the transcripts of both HET and KO animals specifically in genes associated with metabolism and growth. Additionally, metabolic profiling showed increased fat accumulation in HET and KO animals compared with WT, which was increased by a high fat diet. Reduced insulin sensitivity, glucose tolerance, and hyperleptinemia were also observed in HET and KO female mice. Notably, the respiratory exchange ratio of the HET animals was higher than that observed in WT animals, indicating the preferential utilization of glucose as an energy source. These results suggest that the loss of mouse OGA leads to defects in metabolic homeostasis culminating in obesity and insulin resistance.
Keywords: Glycogen Synthase Kinase 3 (GSK-3); Metabolism; Mouse Genetics; O-GlcNAcase; O-GlcNAcylation; O-Linked N-Acetylglucosamine (O-GlcNAc); Perinatal Lethality.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures
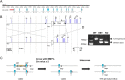

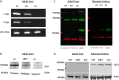
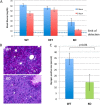
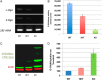

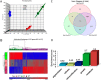
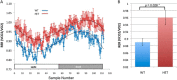



References
-
- Lubas W. A., Frank D. W., Krause M., Hanover J. A. (1997) O-Linked GlcNAc transferase is a conserved nucleocytoplasmic protein containing tetratricopeptide repeats. J. Biol. Chem. 272, 9316–9324 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

