Cytotoxic and natural killer cell stimulatory constituents of Phyllanthus songboiensis
- PMID: 25596805
- PMCID: PMC4333069
- DOI: 10.1016/j.phytochem.2014.12.014
Cytotoxic and natural killer cell stimulatory constituents of Phyllanthus songboiensis
Abstract
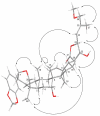
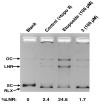
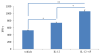
A dichapetalin-type triterpenoid and a dibenzylbutyrolactone-type lignan, together with five known lignans, a known aromatic diterpenoid, and a known acylated phytosterol, were isolated from the aerial parts of Phyllanthus songboiensis, collected in Vietnam. Their structures were determined by interpretation of the spectroscopic data, and the inhibitory activity toward HT-29 human colon cancer cells of all isolates was evaluated by a cytotoxicity assay. The known arylnaphthalene lignan, (+)-acutissimalignan A, was highly cytotoxic toward HT-29 cells, with an IC50 value of 19 nM, but this compound was inactive as a DNA topoisomerase IIα (topo IIα) poison. The known phytosterol, (-)-β-sitosterol-3-O-β-D-(6-O-palmitoyl)glucopyranoside, was found to stimulate natural killer (NK) cells at a concentration of 10μM in the presence of interleukin 12 (IL-12).
Keywords: Cytotoxicity; DNA topoisomerase IIα; Lignan; Natural killer cell; Phyllanthaceae; Phyllanthus songboiensis; Phytosterol.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures

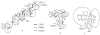
References
-
- Abe F, Yamauchi T. 9α-Hydroxypinoresinol, 9α-hydroxymedioresinol and related lignans from Allamanda neriifolia. Phytochemistry. 1988;27:575–577.
-
- Achenbach H, Asunka SA, Waibel R, Addae-Mensah I, Oppong IV. Dichapetalin A, a novel plant constituent from Dichapetalum madagascariense with potential antineoplastic activity. Nat. Prod. Lett. 1995;7:93–100.
-
- Addae-Mensah I, Waibel R, Asunka SA, Oppong IV, Achenbach H. The dichapetalins-a new class of triterpenoids. Phytochemistry. 1996;43:649–656.
-
- Allerhand A, Maple SR. Requirements for ultrahigh resolution NMR of large molecules on high-field instruments. J. Magn. Reson. 1988;76:375–379.
-
- Belardelli F, Ferrantini M, Proietti E, Kirkwood JM. Interferon-alpha in tumor immunity and immunotherapy. Cytokine Growth Factor Rev. 2002;13:119–134. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

