Intramuscular midazolam versus intravenous lorazepam for the prehospital treatment of status epilepticus in the pediatric population
- PMID: 25597369
- PMCID: PMC4386287
- DOI: 10.1111/epi.12905
Intramuscular midazolam versus intravenous lorazepam for the prehospital treatment of status epilepticus in the pediatric population
Abstract
Objective: To examine the effectiveness of intramuscular (IM) midazolam versus intravenous (IV) lorazepam for the treatment of pediatric patients with status epilepticus (SE) in the prehospital care setting.
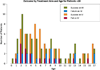
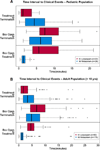
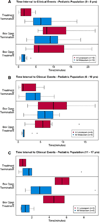
Methods: This multicenter clinical trial randomized patients diagnosed with SE to receive either IM midazolam or IV lorazepam administered by paramedics in the prehospital care setting. Included in this secondary analysis were only patients younger than 18 years of age. Evaluated were the associations of the treatment group (IM vs. IV) with the primary outcome, defined as seizure cessation prior to emergency department (ED) arrival, and with patient characteristics, time to important events, and adverse events. Descriptive statistics and 99% confidence intervals (CIs) were used for the analysis.
Results: Of 893 primary study subjects, 120 met criteria for this study (60 in each treatment group). There were no differences in important baseline characteristics or seizure etiologies between groups. The primary outcome was met in 41 (68.3%) and 43 (71.7%) of subjects in the IM and IV groups, respectively (risk difference [RD] -3.3%, 99% CI -24.9% to 18.2%). Similar results were noted for those younger than 11 years (RD -1.3%, 99% CI -25.7% to 23.1%). Time from initiating the treatment protocol was shorter for children who received IM midazolam, mainly due to the shorter time to administer the active treatment. Safety profiles were similar.
Significance: IM midazolam can be rapidly administered and appears to be safe and effective for the management of children with SE treated in the prehospital setting. The results must be interpreted in the context of the secondary analysis design and sample size of the study.
Keywords: Pediatrics; Prehospital treatment; Status epilepticus.
Wiley Periodicals, Inc. © 2015 International League Against Epilepsy.
Conflict of interest statement
None of the authors has any conflict of interest to disclose. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Figures

References
-
- Brophy GM, Bell R, Claassen J, et al. Guidelines for the evaluation and management of status epilepticus. Neurocrit Care. 2012;17:3–23. - PubMed
-
- Alldredge BK, Gelb AM, Isaacs SM, et al. A comparison of lorazepam, diazepam, and placebo for the treatment of out-of-hospital status epilepticus. N Engl J Med. 2001;345:631–637. - PubMed
-
- Towne AR, DeLorenzo RJ. Use of intramuscular midazolam for status epilepticus. J Emerg Med. 1999;17:323–328. - PubMed
-
- Chamberlain JM, Altieri MA, Futterman C, et al. A prospective, randomized study comparing intramuscular midazolam with intravenous diazepam for the treatment of seizures in children. Pediatr Emerg Care. 1997;13:92–94. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

