The role of the lymphatic system in inflammatory-erosive arthritis
- PMID: 25598390
- PMCID: PMC4397133
- DOI: 10.1016/j.semcdb.2015.01.001
The role of the lymphatic system in inflammatory-erosive arthritis
Abstract
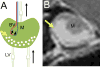
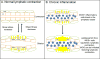
Rheumatoid arthritis (RA) is a prevalent inflammatory joint disease with enigmatic flares, which causes swelling, pain, and irreversible connective tissue damage. Recently, it has been demonstrated in murine models of RA that the popliteal lymph node (PLN) is a biomarker of arthritic flare, as it "expands" in size and contrast enhancement during a prolonged asymptomatic phase, prior to when it "collapses" with accelerated synovitis and joint erosion. This PLN collapse is associated with adjacent knee flare, decreases in PLN volume and contrast enhancement, lymphatic pulse and pumping pressure, and an increase in PLN pressure. Currently, it is known that PLN collapse is accompanied by a translocation of B cells from the follicles to the sinuses, effectively clogging the lymphatic sinuses of the PLN, and that B cell depletion therapy ameliorates arthritic flare by eliminating these B cells and restoring passive lymphatic flow from inflamed joints. Here we review the technological advances that have launched this area of research, describe future directions to help elucidate the potential mechanism of PLN collapse, and speculate on clinical translation towards new diagnostics and therapies for RA.
Keywords: Flare; Lymph node; Lymphatic vessel; Rheumatoid arthritis.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Figures


References
-
- Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003;423:356–61. - PubMed
-
- Nishimura K, Sugiyama D, Kogata Y, Tsuji G, Nakazawa T, Kawano S, et al. Meta-analysis: diagnostic accuracy of anti-cyclic citrullinated peptide antibody and rheumatoid factor for rheumatoid arthritis. Annals of internal medicine. 2007;146:797–808. - PubMed
-
- Feldmann M, Maini SR. Role of cytokines in rheumatoid arthritis: an education in pathophysiology and therapeutics. Immunol Rev. 2008;223:7–19. - PubMed
-
- Nielen MM, van Schaardenburg D, Reesink HW, van de Stadt RJ, van der Horst-Bruinsma IE, de Koning MH, et al. Specific autoantibodies precede the symptoms of rheumatoid arthritis: a study of serial measurements in blood donors. Arthritis Rheum. 2004;50:380–6. - PubMed
Publication types
MeSH terms
Grants and funding
- R21 AR053586/AR/NIAMS NIH HHS/United States
- P30 P30AR061307/AR/NIAMS NIH HHS/United States
- R01 AR063650/AR/NIAMS NIH HHS/United States
- R01 AR056702/AR/NIAMS NIH HHS/United States
- R01 AR048697/AR/NIAMS NIH HHS/United States
- R01 AR056702/AR/NIAMS NIH HHS/United States
- T32 AR053459/AR/NIAMS NIH HHS/United States
- P30 AR061307/AR/NIAMS NIH HHS/United States
- DP2 OD006501/OD/NIH HHS/United States
- DP2OD006501/OD/NIH HHS/United States
- P01 AI078907/AI/NIAID NIH HHS/United States
- 1DP2OD006501-10/OD/NIH HHS/United States
- R01 AR053586/AR/NIAMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

