The long-acting β2 -adrenoceptor agonist, indacaterol, enhances glucocorticoid receptor-mediated transcription in human airway epithelial cells in a gene- and agonist-dependent manner
- PMID: 25598440
- PMCID: PMC4409912
- DOI: 10.1111/bph.13087
The long-acting β2 -adrenoceptor agonist, indacaterol, enhances glucocorticoid receptor-mediated transcription in human airway epithelial cells in a gene- and agonist-dependent manner
Abstract
Background and purpose: Inhaled glucocorticoid (ICS)/long-acting β2 -adrenoceptor agonist (LABA) combination therapy is a recommended treatment option for patients with moderate/severe asthma in whom adequate control cannot be achieved by an ICS alone. Previously, we discovered that LABAs can augment dexamethasone-inducible gene expression and proposed that this effect may explain how these two drugs interact to deliver superior clinical benefit. Herein, we extended that observation by analysing, pharmacodynamically, the effect of the LABA, indacaterol, on glucocorticoid receptor (GR)-mediated gene transcription induced by seven ligands with intrinsic activity values that span the spectrum of full agonism to antagonism.
Experimental approach: BEAS-2B human airway epithelial cells stably transfected with a 2× glucocorticoid response element luciferase reporter were used to model gene transcription together with an analysis of several glucocorticoid-inducible genes.
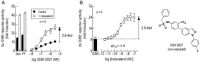
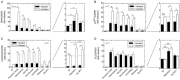
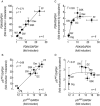
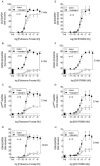
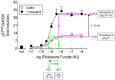
Key results: Indacaterol augmented glucocorticoid-induced reporter activation in a manner that was positively related to the intrinsic activity of the GR agonist. This effect was demonstrated by an increase in response maxima without a change in GR agonist affinity or efficacy. Indacaterol also enhanced glucocorticoid-inducible gene expression. However, the magnitude of this effect was dependent on both the GR agonist and the gene of interest.
Conclusions and implications: These data suggest that indacaterol activates a molecular rheostat, which increases the transcriptional competency of GR in an agonist- and gene-dependent manner without apparently changing the relationship between fractional GR occupancy and response. These findings provide a platform to rationally design ICS/LABA combination therapy that is based on the generation of agonist-dependent gene expression profiles in target and off-target tissues.
© 2015 The British Pharmacological Society.
Figures




Comment in
-
Biased signalling from the glucocorticoid receptor: Renewed opportunity for tailoring glucocorticoid activity.Biochem Pharmacol. 2016 Jul 15;112:6-12. doi: 10.1016/j.bcp.2016.02.008. Epub 2016 Feb 18. Biochem Pharmacol. 2016. PMID: 26898958 Review.
References
-
- Ayroldi E, Riccardi C. Glucocorticoid-induced leucine zipper (GILZ): a new important mediator of glucocorticoid action. FASEB J. 2009;23:3649–3658. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

