Regulation of μ and δ opioid receptor functions: involvement of cyclin-dependent kinase 5
- PMID: 25598508
- PMCID: PMC4409908
- DOI: 10.1111/bph.13088
Regulation of μ and δ opioid receptor functions: involvement of cyclin-dependent kinase 5
Abstract
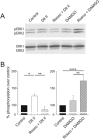
Background and purpose: Phosphorylation of δ opioid receptors (DOP receptors) by cyclin-dependent kinase 5 (CDK5) was shown to regulate the trafficking of this receptor. Therefore, we aimed to determine the role of CDK5 in regulating DOP receptors in rats treated with morphine or with complete Freund's adjuvant (CFA). As μ (MOP) and DOP receptors are known to be co-regulated, we also sought to determine if CDK5-mediated regulation of DOP receptors also affects MOP receptor functions.
Experimental approach: The role of CDK5 in regulating opioid receptors in CFA- and morphine-treated rats was studied using roscovitine as a CDK inhibitor and a cell-penetrant peptide mimicking the second intracellular loop of DOP receptors (C11-DOPri2). Opioid receptor functions were assessed in vivo in a series of behavioural experiments and correlated by measuring ERK1/2 activity in dorsal root ganglia homogenates.
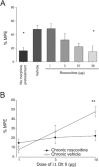
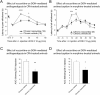
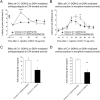
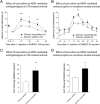
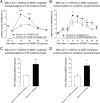
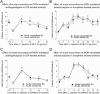
Key results: Chronic roscovitine treatment reduced the antinociceptive and antihyperalgesic effects of deltorphin II (Dlt II) in morphine- and CFA-treated rats respectively. Repeated administrations of C11-DOPri2 also robustly decreased Dlt II-induced analgesia. Interestingly, DAMGO-induced analgesia was significantly increased by roscovitine and C11-DOPri2. Concomitantly, in roscovitine-treated rats the Dlt II-induced ERK1/2 activation was decreased, whereas the DAMGO-induced ERK1/2 activation was increased. An acute roscovitine treatment had no effect on Dlt II- or DAMGO-induced analgesia.
Conclusions and implications: Together, our results demonstrate that CDK5 is a key player in the regulation of DOP receptors in morphine- and CFA-treated rats and that the regulation of DOP receptors by CDK5 is sufficient to modulate MOP receptor functions through an indirect process.
© 2015 The Authors. British Journal of Pharmacology published by John Wiley & Sons Ltd on behalf of The British Pharmacological Society.
Figures
References
-
- Arif A. Extraneuronal activities and regulatory mechanisms of the atypical cyclin-dependent kinase Cdk5. Biochem Pharmacol. 2012;84:985–993. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

