Computational conformational antimicrobial analysis developing mechanomolecular theory for polymer biomaterials in materials science and engineering
- PMID: 25598972
- PMCID: PMC4295723
- DOI: 10.1142/S2047684114500031
Computational conformational antimicrobial analysis developing mechanomolecular theory for polymer biomaterials in materials science and engineering
Abstract
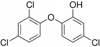
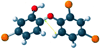
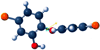
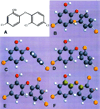
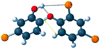
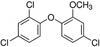
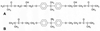
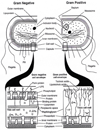
Single-bond rotations or pyramidal inversions tend to either hide or expose relative energies that exist for atoms with nonbonding lone-pair electrons. Availability of lone-pair electrons depends on overall molecular electron distributions and differences in the immediate polarity of the surrounding pico/nanoenvironment. Stereochemistry three-dimensional aspects of molecules provide insight into conformations through single-bond rotations with associated lone-pair electrons on oxygen atoms in addition to pyramidal inversions with nitrogen atoms. When electrons are protected, potential energy is sheltered toward an energy minimum value to compatibilize molecularly with nonpolar environments. When electrons are exposed, maximum energy is available toward polar environment interactions. Computational conformational analysis software calculated energy profiles that exist during specific oxygen ether single-bond rotations with easy-to-visualize three-dimensional models for the trichlorinated bisaromatic ether triclosan antimicrobial polymer additive. As shown, fluctuating alternating bond rotations can produce complex interactions between molecules to provide entanglement strength for polymer toughness or alternatively disrupt weak secondary bonds of attraction to lower resin viscosity for new additive properties with nonpolar triclosan as a hydrophobic toughening/wetting agent. Further, bond rotations involving lone-pair electrons by a molecule at a nonpolar-hydrocarbon-membrane/polar-biologic-fluid interface might become sufficiently unstable to provide free mechanomolecular energies to disrupt weaker microbial membranes, for membrane transport of molecules into cells, provide cell signaling/recognition/defense and also generate enzyme mixing to speed reactions.
Keywords: Conformational analysis; bond rotation; inversion; lone-pair electrons; mechanomolecular; nonpolar; polar.
Figures




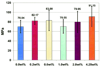
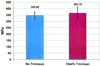
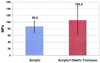
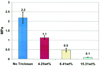

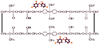
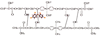







References
-
- Akaki E, Mansur HS, Angelis LH, Castro BA, Valadão HF, Faria DB, Rezende FC. SEM/EDX and FTIR characterization of a dental resin cement with antibacterial agents incorporated. Key Eng. Mater. 2005;284–286:391–394.
-
- Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walters P. Molecular Biology of the Cell. 4th edn. New York: Garland Science; 2002.
-
- Anusavice KJ. Science of Dental Materials. 11th edn. St. Louis: Saunders Elsevier Science; 2003.
-
- Atkins PW. Physical Chemistry. 5th edn. New York: W. H. Freeman and Company; 1994.
-
- Australian Government, Department of Health and Ageing NICNAS. Priority Existing Chemical Assessment Report No. 30, Triclosan. Sydney, Australia: National Industrial Chemical Notification and Assessment Scheme; 2009. http://www.nicnas.gov.au/publications/car/pec/pec30/pec_30_full_report_p....
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
