Physical rehabilitation improves muscle function following volumetric muscle loss injury
- PMID: 25598983
- PMCID: PMC4297368
- DOI: 10.1186/2052-1847-6-41
Physical rehabilitation improves muscle function following volumetric muscle loss injury
Abstract
Background: Given the clinical practice of prescribing physical rehabilitation for the treatment of VML injuries, the present study examined the functional and histomorphological adaptations in the volumetric muscle loss (VML) injured muscle to physical rehabilitation.
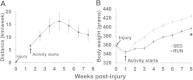
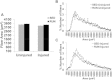
Methods: Tibialis anterior muscle VML injury was created in Lewis rats (n = 32), and were randomly assigned to either sedentary (SED) or physical rehabilitation (RUN) group. After 1 week, RUN rats were given unlimited access to voluntary running wheels either 1 or 7 weeks (2 or 8 weeks post-injury). At 2 weeks post-injury, TA muscles were harvested for molecular analyses. At 8 weeks post-injury, the rats underwent in vivo function testing. The explanted tissue was analyzed using histological and immunofluorescence procedures.
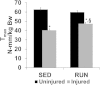
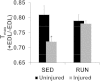
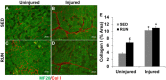
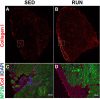
Results: The primary findings of the study are that physical rehabilitation in the form of voluntary wheel running promotes ~ 17% improvement in maximal isometric torque, and a ~ 13% increase in weight of the injured muscle, but it did so without significant morphological adaptations (e.g., no hypertrophy and hyperplasia). Wheel running up-regulated metabolic genes (SIRT-1, PGC-1α) only in the uninjured muscles, and a greater deposition of fibrous tissue in the defect area of the injured muscle preceded by an up-regulation of pro-fibrotic genes (Collagen I, TGF-β1). Therefore, it is plausible that the wheel running related functional improvements were due to improved force transmission and not muscle regeneration.
Conclusions: This is the first study to demonstrate improvement in functional performance of non-repaired VML injured muscle with physical rehabilitation in the form of voluntary wheel running. This study provides information for the first time on the basic changes in the VML injured muscle with physical rehabilitation, which may aid in the development of appropriate physical rehabilitation regimen(s).
Keywords: Function; Muscle; Rehabilitation; Running; Trauma.
Figures



References
-
- Grogan BF, Hsu JR. Volumetric muscle loss. J Am Acad Orthop Surg. 2011;19:S35–S37. - PubMed
-
- Mase VJ, Jr, Hsu JR, Wolf SE, Wenke JC, Baer DG, Owens J, Badylak SF, Walters TJ. Clinical application of an acellular biologic scaffold for surgical repair of a large, traumatic quadriceps femoris muscle defect. Orthopedics. 2010;33(7):511. - PubMed
-
- Machingal MA, Corona BT, Walters TJ, Kesireddy V, Koval CN, Dannahower A, Zhao W, Yoo JJ, Christ GH. A tissue-engineered muscle repair construct for functional restoration of an irrecoverable muscle injury in a murine model. Tissue Eng Part A. 2011;17:2291–2303. doi: 10.1089/ten.tea.2010.0682. - DOI - PMC - PubMed
Pre-publication history
-
- The pre-publication history for this paper can be accessed here: http://www.biomedcentral.com/2052-1847/6/41/prepub
LinkOut - more resources
Full Text Sources
Other Literature Sources

