IFITM-Family Proteins: The Cell's First Line of Antiviral Defense
- PMID: 25599080
- PMCID: PMC4295558
- DOI: 10.1146/annurev-virology-031413-085537
IFITM-Family Proteins: The Cell's First Line of Antiviral Defense
Abstract
Animal cells use a wide variety of mechanisms to slow or prevent replication of viruses. These mechanisms are usually mediated by antiviral proteins whose expression and activities can be constitutive but are frequently amplified by interferon induction. Among these interferon-stimulated proteins, members of the IFITM (interferon-induced transmembrane) family are unique because they prevent infection before a virus can traverse the lipid bilayer of the cell. At least three human IFITM proteins-IFITM1, IFITM2, and IFITM3-have antiviral activities. These activities limit infection in cultured cells by many viruses, including dengue virus, Ebola virus, influenza A virus, severe acute respiratory syndrome coronavirus, and West Nile virus. Murine Ifitm3 controls influenza A virus infection in vivo, and polymorphisms in human IFITM3 correlate with the severity of both seasonal and highly pathogenic avian influenza virus. Here we review the discovery and characterization of the IFITM proteins, describe the spectrum of their antiviral activities, and discuss potential mechanisms underlying these effects.
Keywords: Ebola virus; IFITM3; SARS coronavirus; dengue virus; flavivirus; influenza A virus; interferon; restriction factor; viral entry.
Figures
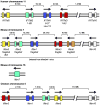

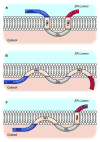
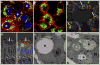
References
-
- Bieniasz PD. Intrinsic immunity: a front-line defense against viral attack. Nat Immunol. 2004;5:1109–15. - PubMed
-
- Friedman RL, Manly SP, McMahon M, Kerr IM, Stark GR. Transcriptional and posttranscriptional regulation of interferon-induced gene expression in human cells. Cell. 1984;38:745–55. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

