Vaccine-induced Human Antibodies Specific for the Third Variable Region of HIV-1 gp120 Impose Immune Pressure on Infecting Viruses
- PMID: 25599085
- PMCID: PMC4293639
- DOI: 10.1016/j.ebiom.2014.10.022
Vaccine-induced Human Antibodies Specific for the Third Variable Region of HIV-1 gp120 Impose Immune Pressure on Infecting Viruses
Abstract
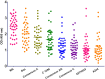
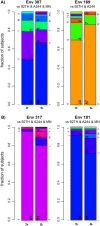
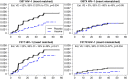
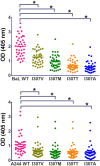
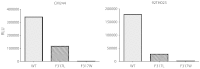
To evaluate the role of V3-specific IgG antibodies (Abs) in the RV144 clinical HIV vaccine trial, which reduced HIV-1 infection by 31.2%, the anti-V3 Ab response was assessed. Vaccinees' V3 Abs were highly cross-reactive with cyclic V3 peptides (cV3s) from diverse virus subtypes. Sieve analysis of CRF01_AE breakthrough viruses from 43 vaccine- and 66 placebo-recipients demonstrated an estimated vaccine efficacy of 85% against viruses with amino acids mismatching the vaccine at V3 site 317 (p=0.004) and 52% against viruses matching the vaccine at V3 site 307 (p=0.004). This analysis was supported by data showing vaccinees' plasma Abs were less reactive with I307 replaced with residues found more often in vaccinees' breakthrough viruses. Simultaneously, viruses with mutations at F317 were less infectious, possibly due to the contribution of F317 to optimal formation of the V3 hydrophobic core. These data suggest that RV144-induced V3-specific Abs imposed immune pressure on infecting viruses and inform efforts to design an HIV vaccine.
Keywords: HIV; antibody; clinical trial; vaccine.
Figures

References
-
- Abagyan R., Batalov S. Homology modeling with internal coordinate mechanics: deformation zone mapping and improvements of models via conformational search. Proteins. 1997;(Suppl. 1):29–37. - PubMed
-
- Andrus L., Prince A.M. Passive immunization with a human immunodeficiency virus type-1 neutralizing monoclonal antibody in Hu-PBL-SCID mice: isolation of a neutralization escape variant. J. Infect. Dis. 1998;177(4):889–897. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

