Seven-membered rings through metal-free rearrangement mediated by hypervalent iodine
- PMID: 25599151
- PMCID: PMC6272645
- DOI: 10.3390/molecules20011475
Seven-membered rings through metal-free rearrangement mediated by hypervalent iodine
Abstract
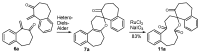
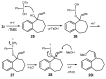
A versatile and metal-free approach for the synthesis of carbocycles and of heterocycles bearing seven- and eight-membered rings is described. The strategy is based on ring expansion of 1-vinylcycloalkanols (or the corresponding silyl or methyl ether) mediated by the hypervalent iodine reagent HTIB (PhI(OH)OTs). Reaction conditions can be easily adjusted to give ring expansion products bearing different functional groups. A route to medium-ring lactones was also developed.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
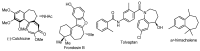
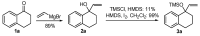




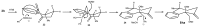
References
-
- Kleinke A.S., Webb D., Jamison T.F. Recent Progress in the Synthesis of Oxepanes and Medium Ring Ethers. Tetrahedron. 2012;68:6999–7018. doi: 10.1016/j.tet.2012.05.081. - DOI
-
- Majumdar K.C. Regioselective Formation of Medium-Ring Heterocycles of Biological Relevance by Intramolecular Cyclization. RSC Adv. 2011;1:1152–1170. doi: 10.1039/c1ra00494h. - DOI
-
- Majumdar K.C., Chattopadhyay B. New Synthetic Strategies for Medium-Sized and Macrocyclic Compounds by Palladium-Catalyzed Cyclization. Curr. Org. Chem. 2009;13:731–757. doi: 10.2174/138527209788167213. - DOI
-
- Chattopadhyay S.K., Karmakar S., Biswas T., Majumdar K.C., Rahaman H., Roy B. Formation of Medium-Ring Heterocycles by Diene and Enyne Metathesis. Tetrahedron. 2007;63:3919–3952. doi: 10.1016/j.tet.2007.01.063. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

