The status of contemporary image-guided modalities in oncologic surgery
- PMID: 25599326
- PMCID: PMC4299947
- DOI: 10.1097/SLA.0000000000000622
The status of contemporary image-guided modalities in oncologic surgery
Abstract
Objective: To review the current trends in optical imaging to guide oncologic surgery.
Background: Surgical resection remains the cornerstone of therapy for patients with early stage solid malignancies and more than half of all patients with cancer undergo surgery each year. The technical ability of the surgeon to obtain clear surgical margins at the initial resection remains crucial to improve overall survival and long-term morbidity. Current resection techniques are largely based on subjective and subtle changes associated with tissue distortion by invasive cancer. As a result, positive surgical margins occur in a significant portion of tumor resections, which is directly correlated with a poor outcome.
Methods: A comprehensive review of studies evaluating optical imaging techniques is performed.
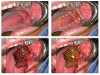
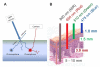
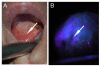
Results: A variety of cancer imaging techniques have been adapted or developed for intraoperative surgical guidance that have been shown to improve functional and oncologic outcomes in randomized clinical trials. There are also a large number of novel, cancer-specific contrast agents that are in early stage clinical trials and preclinical development that demonstrate significant promise to improve real-time detection of subclinical cancer in the operative setting.
Conclusions: There has been an explosion of intraoperative imaging techniques that will become more widespread in the next decade.
Figures



References
-
- Woolgar JA, Triantafyllou A. A histopathological appraisal of surgical margins in oral and oropharyngeal cancer resection specimens. Oral Oncol. 2005;41:1034–43. - PubMed
-
- McMahon J, O’Brien CJ, Pathak I, et al. Influence of condition of surgical margins on local recurrence and disease-specific survival in oral and oropharyngeal cancer. Br J Oral Maxillofac Surg. 2003;41:224–231. - PubMed
-
- Ravasz LA, Slootweg PJ, Hordijk GJ, et al. The status of the resection margin as a prognostic factor in the treatment of head and neck carcinoma. J Craniomaxillofac Surg. 1991;19:314–318. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

