Out-of-hospital mortality among patients receiving methadone for noncancer pain
- PMID: 25599329
- PMCID: PMC4346542
- DOI: 10.1001/jamainternmed.2014.6294
Out-of-hospital mortality among patients receiving methadone for noncancer pain
Abstract
Importance: Growing methadone use in pain management has raised concerns regarding its safety relative to other long-acting opioids. Methadone hydrochloride may increase the risk for lethal respiratory depression related to accidental overdose and life-threatening ventricular arrhythmias.
Objective: To compare the risk of out-of-hospital death in patients receiving methadone for noncancer pain with that in comparable patients receiving sustained-release (SR) morphine sulfate.
Design, setting, and participants: A retrospective cohort study was conducted using Tennessee Medicaid records from 1997 through 2009. The cohort included patients receiving morphine SR or methadone who were aged 30 to 74 years, did not have cancer or another life-threatening illness, and were not in a hospital or nursing home. At cohort entry, 32 742 and 6014 patients had filled a prescription for morphine SR or methadone, respectively. The patients' median age was 48 years, 57.9% were female, and comparable proportions had received cardiovascular, psychotropic, and other musculoskeletal medications. Nearly 90% of the patients received the opioid for back pain or other musculoskeletal pain. The median doses prescribed for morphine SR and methadone were 90 mg/d and 40 mg/d, respectively.
Main outcomes and measures: The primary study end point was out-of-hospital mortality, given that opioid-related deaths typically occur outside the hospital.
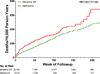
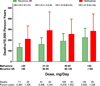
Results: There were 477 deaths during 28 699 person-years of follow-up (ie, 166 deaths per 10 000 person-years). After control for study covariates, patients receiving methadone had a 46% increased risk of death during the follow-up period, with an adjusted hazard ratio (HR) of 1.46 (95% CI, 1.17-1.83; P < .001), resulting in 72 (95% CI, 27-130) excess deaths per 10 000 person-years of follow-up. Methadone doses of 20 mg/d or less, the lowest dose quartile, were associated with an increased risk of death (HR, 1.59; 95% CI, 1.01-2.51, P = .046) relative to a comparable dose of morphine SR (<60 mg/d).
Conclusions and relevance: The increased risk of death observed for patients receiving methadone in this retrospective cohort study, even for low doses, supports recommendations that it should not be a drug of first choice for noncancer pain.
Figures
References
-
- Paulozzi LJ, Mack KA, Jones CM. Vital signs: Risk for overdose from methadone used as pain relief--United States, 1999–2010. Morbidty and Mortality Weekly Report. 2012;61:493–497. - PubMed
-
- Leppert W. The role of methadone in cancer pain treatment - a review. Int J Clin Pract. 2009;63:1095–1109. - PubMed
-
- Fredheim OM, Borchgrevink PC, Kaasa S, Dale O. Clinical pharmacology of methadone for pain. Acta Anaesthesiologica Scandinavica. 2008;52:879–889. - PubMed
-
- Cebert Pharmaceuticals I. Methadone HCL. Columbus, Ohio: Boehringer Ingelheim Roxane, Inc; 2007.
Reference List
-
- Krantz MJ, Martin J, Stimmel B, Mehta D, Haigney MCP. QTc interval screening in methadone treatment. Ann Intern Med. 2009;150:387–395. - PubMed
-
- Pearson EC, Woosley RL. QT prolongation and torsades de pointes among methadone users: reports to the FDA spontaneous reporting system. Pharmacol Drug Safety. 2005;14:747–753. - PubMed
-
- Stringer J, Welsh C, Tommasello A. Methadone-associated Q-T interval prolongation and torsades de pointes. Am J Health-Syst Pharm. 2009;66:825–833. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

