Lineage specificity of primary cilia in the mouse embryo
- PMID: 25599390
- PMCID: PMC4406239
- DOI: 10.1038/ncb3091
Lineage specificity of primary cilia in the mouse embryo
Abstract
Primary cilia are required for vertebrate cells to respond to specific intercellular signals. Here we define when and where primary cilia appear in the mouse embryo using a transgenic line that expresses ARL13B-mCherry in cilia and Centrin 2-GFP in centrosomes. Primary cilia first appear on cells of the epiblast at E6.0 and are subsequently present on all derivatives of the epiblast. In contrast, extraembryonic cells of the visceral endoderm and trophectoderm lineages have centrosomes but no cilia. Stem cell lines derived from embryonic lineages recapitulate the in vivo pattern: epiblast stem cells are ciliated, whereas trophoblast stem cells and extraembryonic endoderm (XEN) stem cells lack cilia. Basal bodies in XEN cells are mature and can form cilia when the AURKA-HDAC6 cilium disassembly pathway is inhibited. The lineage-dependent distribution of cilia is stable throughout much of gestation, defining which cells in the placenta and yolk sac are able to respond to Hedgehog ligands.
Figures
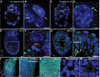
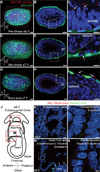
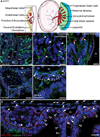
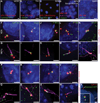


Comment in
-
Control of ciliation in embryogenesis.Nat Cell Biol. 2015 Feb;17(2):109-11. doi: 10.1038/ncb3103. Nat Cell Biol. 2015. PMID: 25633272
References
-
- Caspary T, Larkins CE, Anderson KV. The Graded Response to Sonic Hedgehog Depends on Cilia Architecture. Developmental Cell. 2007;12:767–778. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

