Ring closure activates yeast γTuRC for species-specific microtubule nucleation
- PMID: 25599398
- PMCID: PMC4318760
- DOI: 10.1038/nsmb.2953
Ring closure activates yeast γTuRC for species-specific microtubule nucleation
Abstract
The γ-tubulin ring complex (γTuRC) is the primary microtubule nucleator in cells. γTuRC is assembled from repeating γ-tubulin small complex (γTuSC) subunits and is thought to function as a template by presenting a γ-tubulin ring that mimics microtubule geometry. However, a previous yeast γTuRC structure showed γTuSC in an open conformation that prevents matching to microtubule symmetry. By contrast, we show here that γ-tubulin complexes are in a closed conformation when attached to microtubules. To confirm the functional importance of the closed γTuSC ring, we trapped the closed state and determined its structure, showing that the γ-tubulin ring precisely matches microtubule symmetry and providing detailed insight into γTuRC architecture. Importantly, the closed state is a stronger nucleator, thus suggesting that this conformational switch may allosterically control γTuRC activity. Finally, we demonstrate that γTuRCs have a strong preference for tubulin from the same species.
Figures
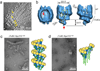
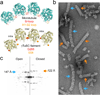



References
-
- Zheng Y, Wong ML, Alberts B, Mitchison T. Nucleation of microtubule assembly by a gamma-tubulin-containing ring complex. Nature. 1995;378:578–583. - PubMed
REFERENCES FOR ONLINE METHODS
-
- Rout MP, Kilmartin JV. Yeast spindle pole body components. Cold Spring Harbor symposia on quantitative biology. 1991;56:687–692. - PubMed
-
- Zheng QS, Braunfeld MB, Sedat JW, Agard DA. An improved strategy for automated electron microscopic tomography. Journal of structural biology. 2004;147:91–101. - PubMed
-
- Förster F, Pruggnaller S, Seybert A, Frangakis AS. Classification of cryo-electron sub-tomograms using constrained correlation. Journal of structural biology. 2008;161:276–286. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

