Effects of testosterone replacement in men with opioid-induced androgen deficiency: a randomized controlled trial
- PMID: 25599449
- PMCID: PMC6036339
- DOI: 10.1097/01.j.pain.0000460308.86819.aa
Effects of testosterone replacement in men with opioid-induced androgen deficiency: a randomized controlled trial
Abstract
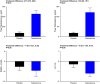
Symptomatic androgen deficiency is common in patients taking opioid analgesics, as these drugs potently suppress the hypothalamic-pituitary-gonadal axis. However, the efficacy of testosterone replacement in this setting remains unclear. The objective of this trial was to evaluate the efficacy of testosterone replacement on pain perception and other androgen-dependent outcomes in men with opioid-induced androgen deficiency. We conducted a randomized, double-blind, parallel placebo-controlled trial at an outpatient academic research center. Participants were men aged 18 to 64 years on opioid analgesics for chronic noncancer pain, and total testosterone levels were <350 ng/dL. Participants were randomly assigned to 14 weeks of daily transdermal gel that contained 5 g of testosterone or placebo. Primary outcomes were changes in self-reported clinical pain and objectively assessed pain sensitivity. Sexual function, quality of life, and body composition were also assessed. The mean age was 49 years. The median total and free testosterone levels at baseline were 243 ng/dL and 47 pg/mL and 251 ng/dL and 43 pg/mL in the testosterone and placebo arm, respectively. Of the 84 randomized participants, 65 had follow-up data on efficacy outcomes. Compared with men assigned to the placebo arm, those assigned to testosterone replacement experienced greater improvements in pressure and mechanical hyperalgesia, sexual desire, and role limitation due to emotional problems. Testosterone administration was also associated with an improvement in body composition. There were no between-group differences in changes in self-reported pain. In conclusion, in men with opioid-induced androgen deficiency, testosterone administration improved pain sensitivity, sexual desire, body composition, and aspects of quality of life.
Trial registration: ClinicalTrials.gov NCT00351819.
Conflict of interest statement
Conflict of interest statement
The remaining authors have no conflicts of interest to declare.
Figures



Comment in
-
Unexplained resolution of diagnosed hypogonadism.Pain. 2016 Apr;157(4):990. doi: 10.1097/j.pain.0000000000000468. Pain. 2016. PMID: 26982515 No abstract available.
-
Reply.Pain. 2016 Apr;157(4):990-991. doi: 10.1097/j.pain.0000000000000467. Pain. 2016. PMID: 26982516 No abstract available.
References
-
- Abs R, Verhelst J, Maeyaert J, Van Buyten JP, Opsomer F, Adriaensen H, Verlooy J, Van Havenbergh T, Smet M, Van Acker K. Endocrine consequences of long-term intrathecal administration of opioids. J Clin Endocrinol Metab. 2000;85:2215–22. - PubMed
-
- Aloisi AM, Ceccarelli I, Fiorenzani P. Gonadectomy affects hormonal and behavioral responses to repetitive nociceptive stimulation in male rats. Ann N Y Acad Sci. 2003;1007:232–7. - PubMed
-
- Arendt-Nielsen L, Yarnitsky D. Experimental and clinical applications of quantitative sensory testing applied to skin, muscles and viscera. J Pain. 2009;10:556–72. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

