Long-term hypoxia increases calcium affinity of BK channels in ovine fetal and adult cerebral artery smooth muscle
- PMID: 25599571
- PMCID: PMC4385992
- DOI: 10.1152/ajpheart.00564.2014
Long-term hypoxia increases calcium affinity of BK channels in ovine fetal and adult cerebral artery smooth muscle
Abstract
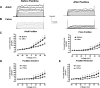
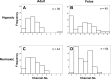
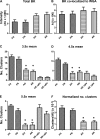
Acclimatization to high-altitude, long-term hypoxia (LTH) reportedly alters cerebral artery contraction-relaxation responses associated with changes in K(+) channel activity. We hypothesized that to maintain oxygenation during LTH, basilar arteries (BA) in the ovine adult and near-term fetus would show increased large-conductance Ca(2+) activated potassium (BK) channel activity. We measured BK channel activity, expression, and cell surface distribution by use of patch-clamp electrophysiology, flow cytometry, and confocal microscopy, respectively, in myocytes from normoxic control and LTH adult and near-term fetus BA. Electrophysiological data showed that BK channels in LTH myocytes exhibited 1) lowered Ca(2+) set points, 2) left-shifted activation voltages, and 3) longer dwell times. BK channels in LTH myocytes also appeared to be more dephosphorylated. These differences collectively make LTH BK channels more sensitive to activation. Studies using flow cytometry showed that the LTH fetus exhibited increased BK β1 subunit surface expression. In addition, in both fetal groups confocal microscopy revealed increased BK channel clustering and colocalization to myocyte lipid rafts. We conclude that increased BK channel activity in LTH BA occurred in association with increased channel affinity for Ca(2+) and left-shifted voltage activation. Increased cerebrovascular BK channel activity may be a mechanism by which LTH adult and near-term fetal sheep can acclimatize to long-term high altitude hypoxia. Our findings suggest that increasing BK channel activity in cerebral myocytes may be a therapeutic target to ameliorate the adverse effects of high altitude in adults or of intrauterine hypoxia in the fetus.
Keywords: Ca2+ signaling; development; high altitude.
Copyright © 2015 the American Physiological Society.
Figures







Similar articles
-
Chronic hypoxia attenuates the vasodilator efficacy of protein kinase G in fetal and adult ovine cerebral arteries.Am J Physiol Heart Circ Physiol. 2017 Jul 1;313(1):H207-H219. doi: 10.1152/ajpheart.00480.2016. Epub 2017 May 26. Am J Physiol Heart Circ Physiol. 2017. PMID: 28550175 Free PMC article.
-
Modulation of BK channel calcium affinity by differential phosphorylation in developing ovine basilar artery myocytes.Am J Physiol Heart Circ Physiol. 2006 Aug;291(2):H732-40. doi: 10.1152/ajpheart.01357.2005. Am J Physiol Heart Circ Physiol. 2006. PMID: 16840736
-
Activation of BKCa channel is associated with increased apoptosis of cerebrovascular smooth muscle cells in simulated microgravity rats.Am J Physiol Cell Physiol. 2010 Jun;298(6):C1489-500. doi: 10.1152/ajpcell.00474.2009. Epub 2010 Mar 24. Am J Physiol Cell Physiol. 2010. PMID: 20457834
-
A BK (Slo1) channel journey from molecule to physiology.Channels (Austin). 2013 Nov-Dec;7(6):442-58. doi: 10.4161/chan.26242. Epub 2013 Sep 11. Channels (Austin). 2013. PMID: 24025517 Free PMC article. Review.
-
Function and regulation of large conductance Ca(2+)-activated K+ channel in vascular smooth muscle cells.Drug Discov Today. 2012 Sep;17(17-18):974-87. doi: 10.1016/j.drudis.2012.04.002. Epub 2012 Apr 13. Drug Discov Today. 2012. PMID: 22521666 Free PMC article. Review.
Cited by
-
Endothelial SK3 channel-associated Ca2+ microdomains modulate blood pressure.Am J Physiol Heart Circ Physiol. 2016 May 1;310(9):H1151-63. doi: 10.1152/ajpheart.00787.2015. Epub 2016 Mar 4. Am J Physiol Heart Circ Physiol. 2016. PMID: 26945080 Free PMC article.
-
Long-term hypoxia modulates depolarization activation of BKCa currents in fetal sheep middle cerebral arterial myocytes.Front Physiol. 2024 Nov 5;15:1479882. doi: 10.3389/fphys.2024.1479882. eCollection 2024. Front Physiol. 2024. PMID: 39563935 Free PMC article.
-
Systemic Hypertension at High Altitude.Hypertension. 2018 Sep;72(3):567-578. doi: 10.1161/HYPERTENSIONAHA.118.11140. Hypertension. 2018. PMID: 30354760 Free PMC article. Review. No abstract available.
-
Role of ryanodine receptor 2 and FK506-binding protein 12.6 dissociation in pulmonary hypertension.J Gen Physiol. 2023 Mar 6;155(3):e202213100. doi: 10.1085/jgp.202213100. Epub 2023 Jan 10. J Gen Physiol. 2023. PMID: 36625865 Free PMC article.
-
Long-Term Hypoxia Negatively Influences Ca2+ Signaling in Basilar Arterial Myocytes of Fetal and Adult Sheep.Front Physiol. 2022 Jan 18;12:760176. doi: 10.3389/fphys.2021.760176. eCollection 2021. Front Physiol. 2022. PMID: 35115953 Free PMC article.
References
-
- Ainslie PN, Subudhi AW. Cerebral blood flow at high altitude. High Alt Med Biol 15: 133–140, 2014. - PubMed
-
- Alioua A, Lu R, Kumar Y, Eghbali M, Kundu P, Toro L, Stefani E. Slo 1 caveolin-binding motif, a mechanism of caveolin-Slo 1 interaction regulating Slo 1 surface expression. J Biol Chem 283: 4809–4817, 2008. - PubMed
-
- Behrens R, Nolting A, Reimann F, Schwarz M, Waldschutz R, Pongs O. hKCNMB3 and hKCNMB4, cloning and characterization of two members of the large-conductance calcium-activated potassium channel beta subunit family. FEBS Lett 474: 99–106, 2000. - PubMed
-
- Berkefeld H, Fakler B, Schulte U. Ca2+-activated K+ channels: from protein complexes to function. Physiol Rev 90: 1437–1459, 2009. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

