Therapeutic window for cyclooxygenase-2 related anti-inflammatory therapy after status epilepticus
- PMID: 25600211
- PMCID: PMC4408774
- DOI: 10.1016/j.nbd.2014.12.032
Therapeutic window for cyclooxygenase-2 related anti-inflammatory therapy after status epilepticus
Abstract
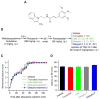
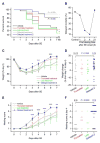
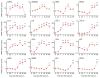
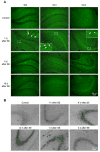
As a prominent inflammatory effector of cyclooxygenase-2 (COX-2), prostaglandin E2 (PGE2) mediates brain inflammation and injury in many chronic central nervous system (CNS) conditions including seizures and epilepsy, largely through its receptor subtype EP2. However, EP2 receptor activation might also be neuroprotective in models of excitotoxicity and ischemia. These seemingly incongruent observations expose the delicacy of immune and inflammatory signaling in the brain; thus the therapeutic window for quelling neuroinflammation might vary with injury type and target molecule. Here, we identify a therapeutic window for EP2 antagonism to reduce delayed mortality and functional morbidity after status epilepticus (SE) in mice. Importantly, treatment must be delayed relative to SE onset to be effective, a finding that could be explained by the time-course of COX-2 induction after SE and compound pharmacokinetics. A large number of inflammatory mediators were upregulated in hippocampus after SE with COX-2 and IL-1β temporally leading many others. Thus, EP2 antagonism represents a novel anti-inflammatory strategy to treat SE with a tightly-regulated therapeutic window.
Keywords: Chemokine; Cyclooxygenase; Cytokine; EP2; Epilepsy; Gliosis; Interleukin-1β; Neuroinflammation; Prostaglandin; Seizure; Status epilepticus.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures


References
-
- Aronica E, Crino PB. Inflammation in epilepsy: clinical observations. Epilepsia. 2011;52(Suppl 3):26–32. - PubMed
-
- Balosso S, Ravizza T, Aronica E, Vezzani A. The dual role of TNF-alpha and its receptors in seizures. Exp Neurol. 2013;247:267–71. - PubMed
-
- Bazan NG. COX-2 as a multifunctional neuronal modulator. Nat Med. 2001;7:414–5. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

