Insights into the trimeric HIV-1 envelope glycoprotein structure
- PMID: 25600289
- PMCID: PMC4310573
- DOI: 10.1016/j.tibs.2014.12.006
Insights into the trimeric HIV-1 envelope glycoprotein structure
Abstract
The HIV-1 envelope glycoprotein (Env) trimer is responsible for receptor recognition and viral fusion with CD4(+) T cells, and is the sole target for neutralizing antibodies. Thus, understanding its molecular architecture is of significant interest. However, the Env trimer has proved to be a challenging target for 3D structure determination. Recent electron microscopy (EM) and X-ray structures have at last enabled us to decipher the structural complexity and unique features of the Env trimer, and how it is recognized by an ever-expanding arsenal of potent broadly neutralizing antibodies. We describe our current knowledge of the Env trimer structure in the context of exciting recent developments in the identification and characterization of HIV broadly neutralizing antibodies.
Keywords: HIV-1; broadly neutralizing antibodies; envelope glycoprotein trimer; structure.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Figures


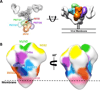
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

