The osteogenic niche promotes early-stage bone colonization of disseminated breast cancer cells
- PMID: 25600338
- PMCID: PMC4326554
- DOI: 10.1016/j.ccell.2014.11.017
The osteogenic niche promotes early-stage bone colonization of disseminated breast cancer cells
Abstract
Breast cancer bone micrometastases can remain asymptomatic for years before progressing into overt lesions. The biology of this process, including the microenvironment niche and supporting pathways, is unclear. We find that bone micrometastases predominantly reside in a niche that exhibits features of osteogenesis. Niche interactions are mediated by heterotypic adherens junctions (hAJs) involving cancer-derived E-cadherin and osteogenic N-cadherin, the disruption of which abolishes niche-conferred advantages. We elucidate that hAJ activates the mTOR pathway in cancer cells, which drives the progression from single cells to micrometastases. Human data set analyses support the roles of AJ and the mTOR pathway in bone colonization. Our study illuminates the initiation of bone colonization, and provides potential therapeutic targets to block progression toward osteolytic metastases.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures

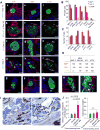

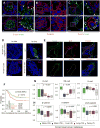
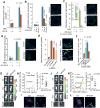
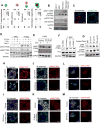

Comment in
-
Cradle of evil: osteogenic niche for early bone metastasis.Cancer Cell. 2015 Feb 9;27(2):153-5. doi: 10.1016/j.ccell.2015.01.006. Cancer Cell. 2015. PMID: 25670074
References
-
- Baron R, Kneissel M. WNT signaling in bone homeostasis and disease: from human mutations to treatments. Nat Med. 2013;19:179–192. - PubMed
-
- Boulay A, Rudloff J, Ye J, Zumstein-Mecker S, O'Reilly T, Evans DB, Chen S, Lane HA. Dual inhibition of mTOR and estrogen receptor signaling in vitro induces cell death in models of breast cancer. Clin Cancer Res. 2005;11:5319–5328. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

