Immunodeficiency-related vaccine-derived poliovirus (iVDPV) cases: a systematic review and implications for polio eradication
- PMID: 25600519
- PMCID: PMC5529169
- DOI: 10.1016/j.vaccine.2015.01.018
Immunodeficiency-related vaccine-derived poliovirus (iVDPV) cases: a systematic review and implications for polio eradication
Abstract
Background: Vaccine-derived polioviruses (VDPVs), strains of poliovirus mutated from the oral polio vaccine, pose a challenge to global polio eradication. Immunodeficiency-related vaccine-derived polioviruses (iVDPVs) are a type of VDPV which may serve as sources of poliovirus reintroduction after the eradication of wild-type poliovirus. This review is a comprehensive update of confirmed iVDPV cases published in the scientific literature from 1962 to 2012, and describes clinically relevant trends in reported iVDPV cases worldwide.
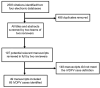
Methods: We conducted a systematic review of published iVDPV case reports from January 1960 to November 2012 from four databases. We included cases in which the patient had a primary immunodeficiency, and the vaccine virus isolated from the patient either met the sequencing definition of VDPV (>1% divergence for serotypes 1 and 3 and >0.6% for serotype 2) and/or was previously reported as an iVDPV by the World Health Organization.
Results: We identified 68 iVDPV cases in 49 manuscripts reported from 25 countries and the Palestinian territories. 62% of case patients were male, 78% presented clinically with acute flaccid paralysis, and 65% were iVDPV2. 57% of cases occurred in patients with predominantly antibody immunodeficiencies, and the overall all-cause mortality rate was greater than 60%. The median age at case detection was 1.4 years [IQR: 0.8, 4.5] and the median duration of shedding was 1.3 years [IQR: 0.7, 2.2]. We identified a poliovirus genome VP1 region mutation rate of 0.72% per year and a higher median percent divergence for iVDPV1 cases. More cases were reported from high income countries, which also had a larger age variation and different distribution of immunodeficiencies compared to upper and lower middle-income countries.
Conclusion: Our study describes the incidence and characteristics of global iVDPV cases reported in the literature in the past five decades. It also highlights the regional and economic disparities of reported iVDPV cases.
Keywords: Immunodeficiency; Oral polio vaccine; Polio eradication; Poliomyelitis; iVDPV.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Figures




References
-
- Liu X, Levin A, Makinen M, Day J. OPV vs IPV: past and future choice of vaccine in the global polio eradication program. Partn Health Reform Proj. 2003
-
- Heymann DL, Sutter RW, Aylward RB. A vision of a world without polio: the OPV cessation strategy. Biologicals. 2006;34(2):75–9. - PubMed
-
- Aylward B, Yamada T. The polio endgame. N Engl J Med. 2011;364(24):2273–5. - PubMed
-
- Nathanson N. Eradication of poliovirus: fighting fire with fire. J Infect Dis. 2011;203(7):889–90. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

