Structure and function of serotonin G protein-coupled receptors
- PMID: 25601315
- PMCID: PMC4414735
- DOI: 10.1016/j.pharmthera.2015.01.009
Structure and function of serotonin G protein-coupled receptors
Abstract
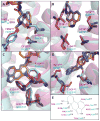
Serotonin receptors are prevalent throughout the nervous system and the periphery, and remain one of the most lucrative and promising drug discovery targets for disorders ranging from migraine headaches to neuropsychiatric disorders such as schizophrenia and depression. There are 14 distinct serotonin receptors, of which 13 are G protein-coupled receptors (GPCRs), which are targets for approximately 40% of the approved medicines. Recent crystallographic and biochemical evidence has provided a converging understanding of the basic structure and functional mechanics of GPCR activation. Currently, two GPCR crystal structures exist for the serotonin family, the 5-HT1B and 5-HT2B receptor, with the antimigraine and valvulopathic drug ergotamine bound. The first serotonin crystal structures not only provide the first evidence of serotonin receptor topography but also provide mechanistic explanations into functional selectivity or biased agonism. This review will detail the findings of these crystal structures from a molecular and mutagenesis perspective for driving rational drug design for novel therapeutics incorporating biased signaling.
Keywords: 5-HT(1B); 5-HT(2B); Functional selectivity; GPCR; Serotonin; β-Arrestin.
Copyright © 2015 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures


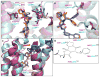


References
-
- Adham N, Romanienko P, Hartig P, Weinshank RL, Branchek T. The Rat 5-Hydroxytryptamine1B Receptor Is the Species Homologue of the Human 5-Hydroxytryptamine1D Beta Receptor. Mol Pharmacol. 1992;41:1–7. - PubMed
-
- Allen JA, Roth BL. Strategies to Discover Unexpected Targets for Drugs Active at G Protein-Coupled Receptors. Annu Rev Pharmacol Toxicol. 2011;51:117–144. - PubMed
-
- Almaula N, Ebersole BJ, Ballesteros JA, Weinstein H, Sealfon SC. Contribution of a Helix 5 Locus to Selectivity of Hallucinogenic and Nonhallucinogenic Ligands for the Human 5-Hydroxytryptamine2A and 5-Hydroxytryptamine2C Receptors: Direct and Indirect Effects on Ligand Affinity Mediated by the Same Locus. Mol Pharmacol. 1996a;50:34–42. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

