Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study
- PMID: 25601342
- PMCID: PMC4419359
- DOI: 10.1016/S1470-2045(14)71207-0
Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study
Abstract
Background: We aimed to compare overall survival after standard-dose versus high-dose conformal radiotherapy with concurrent chemotherapy and the addition of cetuximab to concurrent chemoradiation for patients with inoperable stage III non-small-cell lung cancer.
Methods: In this open-label randomised, two-by-two factorial phase 3 study in 185 institutions in the USA and Canada, we enrolled patients (aged ≥ 18 years) with unresectable stage III non-small-cell lung cancer, a Zubrod performance status of 0-1, adequate pulmonary function, and no evidence of supraclavicular or contralateral hilar adenopathy. We randomly assigned (1:1:1:1) patients to receive either 60 Gy (standard dose), 74 Gy (high dose), 60 Gy plus cetuximab, or 74 Gy plus cetuximab. All patients also received concurrent chemotherapy with 45 mg/m(2) paclitaxel and carboplatin once a week (AUC 2); 2 weeks after chemoradiation, two cycles of consolidation chemotherapy separated by 3 weeks were given consisting of paclitaxel (200 mg/m(2)) and carboplatin (AUC 6). Randomisation was done with permuted block randomisation methods, stratified by radiotherapy technique, Zubrod performance status, use of PET during staging, and histology; treatment group assignments were not masked. Radiation dose was prescribed to the planning target volume and was given in 2 Gy daily fractions with either intensity-modulated radiation therapy or three-dimensional conformal radiation therapy. The use of four-dimensional CT and image-guided radiation therapy were encouraged but not necessary. For patients assigned to receive cetuximab, 400 mg/m(2) cetuximab was given on day 1 followed by weekly doses of 250 mg/m(2), and was continued through consolidation therapy. The primary endpoint was overall survival. All analyses were done by modified intention-to-treat. The study is registered with ClinicalTrials.gov, number NCT00533949.
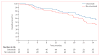
Findings: Between Nov 27, 2007, and Nov 22, 2011, 166 patients were randomly assigned to receive standard-dose chemoradiotherapy, 121 to high-dose chemoradiotherapy, 147 to standard-dose chemoradiotherapy and cetuximab, and 110 to high-dose chemoradiotherapy and cetuximab. Median follow-up for the radiotherapy comparison was 22.9 months (IQR 27.5-33.3). Median overall survival was 28.7 months (95% CI 24.1-36.9) for patients who received standard-dose radiotherapy and 20.3 months (17.7-25.0) for those who received high-dose radiotherapy (hazard ratio [HR] 1.38, 95% CI 1.09-1.76; p=0.004). Median follow-up for the cetuximab comparison was 21.3 months (IQR 23.5-29.8). Median overall survival in patients who received cetuximab was 25.0 months (95% CI 20.2-30.5) compared with 24.0 months (19.8-28.6) in those who did not (HR 1.07, 95% CI 0.84-1.35; p=0.29). Both the radiation-dose and cetuximab results crossed protocol-specified futility boundaries. We recorded no statistical differences in grade 3 or worse toxic effects between radiotherapy groups. By contrast, the use of cetuximab was associated with a higher rate of grade 3 or worse toxic effects (205 [86%] of 237 vs 160 [70%] of 228 patients; p<0.0001). There were more treatment-related deaths in the high-dose chemoradiotherapy and cetuximab groups (radiotherapy comparison: eight vs three patients; cetuximab comparison: ten vs five patients). There were no differences in severe pulmonary events between treatment groups. Severe oesophagitis was more common in patients who received high-dose chemoradiotherapy than in those who received standard-dose treatment (43 [21%] of 207 patients vs 16 [7%] of 217 patients; p<0.0001).
Interpretation: 74 Gy radiation given in 2 Gy fractions with concurrent chemotherapy was not better than 60 Gy plus concurrent chemotherapy for patients with stage III non-small-cell lung cancer, and might be potentially harmful. Addition of cetuximab to concurrent chemoradiation and consolidation treatment provided no benefit in overall survival for these patients.
Funding: National Cancer Institute and Bristol-Myers Squibb.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Conflict of interest statement
RP reports grants from National Cancer Institute, grants from Bristol-Myers Squibb, during the conduct of the study; GB reports personal fees and non-financial support from Bristol-Myers Squibb, during the conduct of the study, personal fees from Bayer Healthcare, Biothera Pharma, and Novartis Pharmaceuticals outside the submitted work; CH reports grants from Bristol-Myers Squibb (U10CA21661 U10CA37422) and funds for general study support (Bristol-Myers Squibb did not at any time have the right or were they permitted to effect underlying analysis or edit any portion of this manuscript); AM has a consultant or advisory role for Ventana Medical System, receives honoraria from Ventana Medical Systems and Genoptix, and received research funding from Roche and Ventana Medical Systems; HC has a consulting or advisory role for Bayer and EMD Serono, and receives research funding from Celgene (institution); JDB, RK, GM, SS, JBo, KF, VN, YIG, SN, PI, CR, RBW, CK, JM, JBe, RG, and WC declare no competing interests.
Figures


Comment in
-
Dose escalation in lung cancer: have we gone full circle?Lancet Oncol. 2015 Feb;16(2):125-7. doi: 10.1016/S1470-2045(15)70001-X. Epub 2015 Jan 16. Lancet Oncol. 2015. PMID: 25601340 No abstract available.
-
Radiotherapy dose and fractionation for stage III NSCLC.Lancet Oncol. 2015 Apr;16(4):e156-7. doi: 10.1016/S1470-2045(15)70121-X. Lancet Oncol. 2015. PMID: 25846093 No abstract available.
References
-
- Perez CA, Stanley K, Rubin P, et al. A prospective randomized study of various irradiation doses and fractionation schedules in the treatment of inoperable non-oat-cell carcinoma of the lung. Preliminary report by the Radiation Therapy Oncology Group. Cancer. 1980;45:2744–53. - PubMed
-
- Bradley JD, Bae K, Graham MV, et al. Primary analysis of the phase II component of a phase I/II dose intensification study using three-dimensional conformal radiation therapy and concurrent chemotherapy for patients with inoperable non-small-cell lung cancer: RTOG 0117. J Clin Oncol. 2010;28:2475–80. - PMC - PubMed
-
- Schild SE, McGinnis WL, Graham D, et al. Results of a Phase I trial of concurrent chemotherapy and escalating doses of radiation for unresectable non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2006;65:1106–11. - PubMed
-
- Socinski MA, Blackstock AW, Bogart JA, et al. Randomized phase II trial of induction chemotherapy followed by concurrent chemotherapy and dose-escalated thoracic conformal radiotherapy (74 Gy) in stage III non-small-cell lung cancer: CALGB 30105. J Clin Oncol. 2008;26:2457–63. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

