Histopathological, biomechanical, and behavioral pain findings of Achilles tendinopathy using an animal model of overuse injury
- PMID: 25602018
- PMCID: PMC4387767
- DOI: 10.14814/phy2.12265
Histopathological, biomechanical, and behavioral pain findings of Achilles tendinopathy using an animal model of overuse injury
Abstract
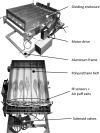
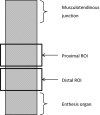
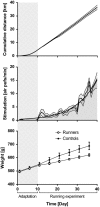
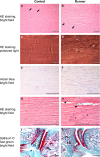
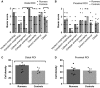
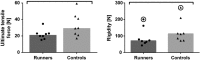
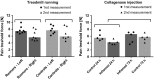
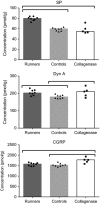
Animal models of forced running are used to study overuse tendinopathy, a common health problem for which clear evidence for effective and accessible treatments is still lacking. In these models, pain evaluation is necessary to better understand the disease, help design and evaluate therapies, and ensure humane treatment of the animals. Therefore, the main objective of this study was to evaluate pain and pathologic findings in an animal model of moderate Achilles tendinopathy induced by treadmill running. Air puffs, instead of electrical shocks, were used to stimulate running so that pain associated with stimulation would be avoided. Pressure pain sensitivity was evaluated in vivo using a new instrumented plier, whereas spinal cord peptides were analyzed ex vivo with high-performance liquid chromatography tandem mass spectrometry. Tendon histologic slides were semiquantitatively evaluated, using the Bonar score technique and biomechanical properties, using the traction test. After 8 weeks of treadmill running (2 weeks for adaptation and 6 weeks for the lesion protocol), the protocol was stopped because the air puffs became ineffective to stimulate running. We, nevertheless, observed some histologic changes characteristic of overuse tendinopathy as well as decreased mechanical properties, increased Substance P and dynorphin A peptides but without pressure pain sensitivity. These results suggest that air-puffs stimulation is sufficient to induce an early stage tendinopathy to study new therapeutic drugs without inducing unnecessary pain. They also indicate that pain-associated peptides could be related with movement evoked pain and with the sharp breakdown of the running performance.
Keywords: Air‐puff stimulation; Bonar score; Randall–Selitto test; calcitonin gene‐related peptide; dynorphin A; instrumented plier; mechanical properties; substance P; treadmill.
© 2015 The Authors. Physiological Reports published by Wiley Periodicals, Inc. on behalf of the American Physiological Society and The Physiological Society.
Figures

Similar articles
-
The effect of dry needling and treadmill running on inducing pathological changes in rat Achilles tendon.Connect Tissue Res. 2015 Nov;56(6):452-60. doi: 10.3109/03008207.2015.1052876. Epub 2015 Jul 29. Connect Tissue Res. 2015. PMID: 26076317
-
Continued sports activity, using a pain-monitoring model, during rehabilitation in patients with Achilles tendinopathy: a randomized controlled study.Am J Sports Med. 2007 Jun;35(6):897-906. doi: 10.1177/0363546506298279. Epub 2007 Feb 16. Am J Sports Med. 2007. PMID: 17307888 Clinical Trial.
-
Uphill running improves rat Achilles tendon tissue mechanical properties and alters gene expression without inducing pathological changes.J Appl Physiol (1985). 2012 Sep 1;113(5):827-36. doi: 10.1152/japplphysiol.00401.2012. Epub 2012 Jul 12. J Appl Physiol (1985). 2012. PMID: 22797314
-
Achilles tendinitis in running athletes.J Am Board Fam Pract. 1989 Jul-Sep;2(3):196-203. J Am Board Fam Pract. 1989. PMID: 2665426 Review.
-
Imaging in the assessment and management of Achilles tendinopathy and paratendinitis.Semin Musculoskelet Radiol. 2011 Feb;15(1):89-100. doi: 10.1055/s-0031-1271961. Epub 2011 Feb 17. Semin Musculoskelet Radiol. 2011. PMID: 21332022 Review.
Cited by
-
Sustained Exposure of Substance P Causes Tendinopathy.Int J Mol Sci. 2020 Nov 16;21(22):8633. doi: 10.3390/ijms21228633. Int J Mol Sci. 2020. PMID: 33207770 Free PMC article.
-
Collagen denaturation in post-run Achilles tendons and Achilles tendinopathy: In vivo mechanophysiology and magnetic resonance imaging.Sci Adv. 2024 Oct 4;10(40):eado2015. doi: 10.1126/sciadv.ado2015. Epub 2024 Oct 2. Sci Adv. 2024. PMID: 39356750 Free PMC article.
-
Advances in Microscopic Studies of Tendinopathy: Literature Review and Current Trends, with Special Reference to Neovascularization Process.J Clin Med. 2022 Mar 13;11(6):1572. doi: 10.3390/jcm11061572. J Clin Med. 2022. PMID: 35329898 Free PMC article. Review.
-
Tendinopathy: Investigating the Intersection of Clinical and Animal Research to Identify Progress and Hurdles in the Field.JBJS Rev. 2016 Oct 11;4(10):01874474-201610000-00004. doi: 10.2106/JBJS.RVW.15.00088. JBJS Rev. 2016. PMID: 27792676 Free PMC article.
-
The analgesic effect of joint mobilization and manipulation in tendinopathy: a narrative review.J Man Manip Ther. 2021 Oct;29(5):276-287. doi: 10.1080/10669817.2021.1904348. Epub 2021 Mar 26. J Man Manip Ther. 2021. PMID: 33769226 Free PMC article. Review.
References
-
- Ackermann P. W., Finn A., Ahmed M. 1999. Sensory neuropeptidergic pattern in tendon, ligament and joint capsule. A study in the rat. NeuroReport; 10:2055-2060. - PubMed
-
- Alfredson H., Thorsen K., Lorentzon R. 1999. In situ microdialysis in tendon tissue: high levels of glutamate, but not prostaglandin E2 in chronic Achilles tendon pain. Knee Surg. Sports Traumatol. Arthrosc.; 7:378-381. - PubMed
-
- Alfredson H., Bjur D., Thorsen K., Lorentzon R., Sandström P. 2002. High intratendinous lactate levels in painful chronic Achilles tendinosis. An investigation using microdialysis technique. J. Orthop. Res.; 20:934-938. - PubMed
-
- Almekinders L., Banes A. J. 2007. 160-169inIn: Woo S. L.‐Y., H Renström P. A. F., Arnoczky S. P. (eds.). An integrative therapeutic approach to tendinopathy: biomechanicas and biological considerations. Tendinopathy in athletes, The encyclopedia of sports medicine XII Malden, MA: Blackwell Pub
LinkOut - more resources
Full Text Sources
Other Literature Sources

