The role and interaction of imprinted genes in human fetal growth
- PMID: 25602077
- PMCID: PMC4305174
- DOI: 10.1098/rstb.2014.0074
The role and interaction of imprinted genes in human fetal growth
Abstract
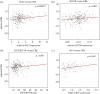
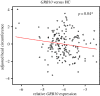
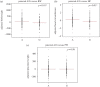
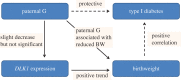
Identifying the genetic input for fetal growth will help to understand common, serious complications of pregnancy such as fetal growth restriction. Genomic imprinting is an epigenetic process that silences one parental allele, resulting in monoallelic expression. Imprinted genes are important in mammalian fetal growth and development. Evidence has emerged showing that genes that are paternally expressed promote fetal growth, whereas maternally expressed genes suppress growth. We have assessed whether the expression levels of key imprinted genes correlate with fetal growth parameters during pregnancy, either early in gestation, using chorionic villus samples (CVS), or in term placenta. We have found that the expression of paternally expressing insulin-like growth factor 2 (IGF2), its receptor IGF2R, and the IGF2/IGF1R ratio in CVS tissues significantly correlate with crown-rump length and birthweight, whereas term placenta expression shows no correlation. For the maternally expressing pleckstrin homology-like domain family A, member 2 (PHLDA2), there is no correlation early in pregnancy in CVS but a highly significant negative relationship in term placenta. Analysis of the control of imprinted expression of PHLDA2 gave rise to a maternally and compounded grand-maternally controlled genetic effect with a birthweight increase of 93/155 g, respectively, when one copy of the PHLDA2 promoter variant is inherited. Expression of the growth factor receptor-bound protein 10 (GRB10) in term placenta is significantly negatively correlated with head circumference. Analysis of the paternally expressing delta-like 1 homologue (DLK1) shows that the paternal transmission of type 1 diabetes protective G allele of rs941576 single nucleotide polymorphism (SNP) results in significantly reduced birth weight (-132 g). In conclusion, we have found that the expression of key imprinted genes show a strong correlation with fetal growth and that for both genetic and genomics data analyses, it is important not to overlook parent-of-origin effects.
Keywords: birth weight; chorionic villus sampling; fetal growth restriction; genomic imprinting; placenta; type 1 diabetes.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

